Input interpretation

magnesium perchlorate | selenium trioxide
Chemical names and formulas
| magnesium perchlorate | selenium trioxide formula | Mg(ClO_4)_2 | SeO_3 Hill formula | Cl_2MgO_8 | O_3Se name | magnesium perchlorate | selenium trioxide IUPAC name | magnesium diperchlorate | alternate names | anhydrone | anhydrous magnesium perchlorate | dehydrite | perchloric acid magnesium salt | selenic anhydride mass fractions | Cl (chlorine) 31.8% | Mg (magnesium) 10.9% | O (oxygen) 57.3% | O (oxygen) 37.8% | Se (selenium) 62.2%
Structure diagrams
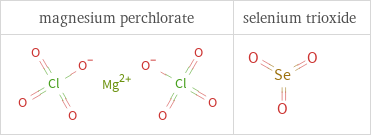
Structure diagrams
Basic properties
| magnesium perchlorate | selenium trioxide molar mass | 223.2 g/mol | 126.97 g/mol phase | solid (at STP) | solid (at STP) melting point | 251 °C | 118.35 °C density | 2.21 g/cm^3 | 3.6 g/cm^3 solubility in water | | very soluble
Units

Solid properties (at STP)

| magnesium perchlorate | selenium trioxide density | 2.21 g/cm^3 | 3.6 g/cm^3
Units
