Input interpretation
magnesium hydroxide | barium iron oxide
Chemical names and formulas
![| magnesium hydroxide | barium iron oxide formula | Mg(OH)_2 | BaFe_12O_19 Hill formula | H_2MgO_2 | BaFe_12O_19 name | magnesium hydroxide | barium iron oxide IUPAC name | magnesium dihydroxide | oxobarium; oxo-(oxoferriooxy)iron alternate names | magnesia magma | magnesia, [Milk of] | magnesium dihydroxide | magnesium hydrate | milk of magnesia | barium dodecairon nonadecaoxide | barium ferrate | barium ferrite | barium hexaferrite | ferroxdure | ketobarium; keto-(ketoferriooxy)iron | oxobarium; oxo-(oxoferriooxy)iron mass fractions | H (hydrogen) 3.46% | Mg (magnesium) 41.7% | O (oxygen) 54.9% | Ba (barium) 12.4% | Fe (iron) 60.3% | O (oxygen) 27.3%](../image_source/92a6d18b71ca33c9cd8469664dce90d9.png)
| magnesium hydroxide | barium iron oxide formula | Mg(OH)_2 | BaFe_12O_19 Hill formula | H_2MgO_2 | BaFe_12O_19 name | magnesium hydroxide | barium iron oxide IUPAC name | magnesium dihydroxide | oxobarium; oxo-(oxoferriooxy)iron alternate names | magnesia magma | magnesia, [Milk of] | magnesium dihydroxide | magnesium hydrate | milk of magnesia | barium dodecairon nonadecaoxide | barium ferrate | barium ferrite | barium hexaferrite | ferroxdure | ketobarium; keto-(ketoferriooxy)iron | oxobarium; oxo-(oxoferriooxy)iron mass fractions | H (hydrogen) 3.46% | Mg (magnesium) 41.7% | O (oxygen) 54.9% | Ba (barium) 12.4% | Fe (iron) 60.3% | O (oxygen) 27.3%
Structure diagrams
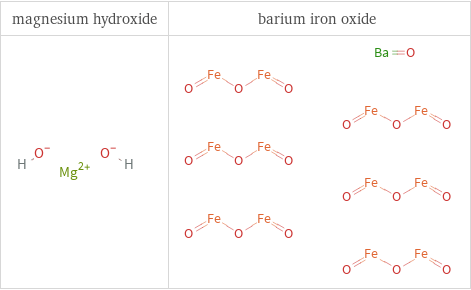
Structure diagrams
Basic properties

| magnesium hydroxide | barium iron oxide molar mass | 58.319 g/mol | 1111.4 g/mol phase | solid (at STP) | melting point | 350 °C | density | 2.3446 g/cm^3 | 5.28 g/cm^3 solubility in water | insoluble | particle size | 100 nm | 100 nm
Solid properties
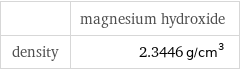
| magnesium hydroxide density | 2.3446 g/cm^3
Units
