Input interpretation
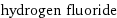
hydrogen fluoride
Chemical names and formulas

formula | HF Hill formula | FH name | hydrogen fluoride alternate names | anhydrous hydrofluoric acid | anhydrous hydrogen fluoride | fluorane | hydrofluoride | hydron fluoride | rubigine mass fractions | F (fluorine) 95% | H (hydrogen) 5.04%
Structure diagram

Structure diagram
Basic properties
molar mass | 20.006 g/mol phase | gas (at STP) melting point | -83.36 °C boiling point | 19.5 °C density | 8.18×10^-4 g/cm^3 (at 25 °C) solubility in water | miscible
Units

Gas properties (at STP)
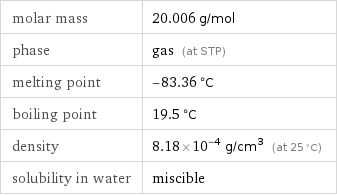
density | 8.18×10^-4 g/cm^3 (at 25 °C) vapor density | 1.27 (relative to air) molar volume | 24500 cm^3/mol refractive index | 1.00001 dynamic viscosity | 1.2571×10^-5 Pa s (at 20 °C)
Units

Thermodynamic properties
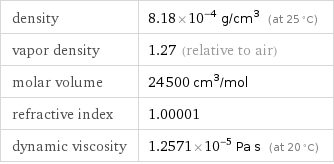
specific free energy of formation Δ_fG° | gas | -13.77 kJ/g molar free energy of formation Δ_fG° | gas | -275.4 kJ/mol specific heat of formation Δ_fH° | gas | -13.66 kJ/g | liquid | -14.99 kJ/g molar heat of formation Δ_fH° | gas | -273.3 kJ/mol | liquid | -299.8 kJ/mol specific entropy S° | gas | 8.687 J/(g K) molar entropy S° | gas | 173.8 J/(mol K) molar heat of vaporization | 7.5 kJ/mol | specific heat of vaporization | 0.37 kJ/g | molar heat of fusion | 4.58 kJ/mol | specific heat of fusion | 0.229 kJ/g | critical temperature | 461 K | critical pressure | 6.48 MPa | (at STP)
Chemical identifiers
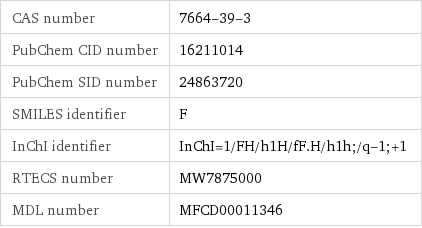
CAS number | 7664-39-3 PubChem CID number | 16211014 PubChem SID number | 24863720 SMILES identifier | F InChI identifier | InChI=1/FH/h1H/fF.H/h1h;/q-1;+1 RTECS number | MW7875000 MDL number | MFCD00011346
NFPA label

NFPA label

NFPA health rating | 4 NFPA fire rating | 0 NFPA reactivity rating | 1
Toxicity properties

short-term exposure limit | 2.5 mg/m^3
Units

Ion equivalents

H^+ (hydrogen cation) | 1 F^- (fluoride anion) | 1