Input interpretation
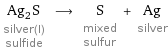
Ag_2S silver(I) sulfide ⟶ S mixed sulfur + Ag silver
Chemical names and formulas
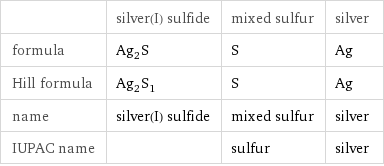
| silver(I) sulfide | mixed sulfur | silver formula | Ag_2S | S | Ag Hill formula | Ag_2S_1 | S | Ag name | silver(I) sulfide | mixed sulfur | silver IUPAC name | | sulfur | silver
Substance properties
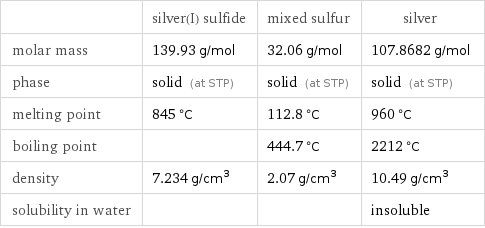
| silver(I) sulfide | mixed sulfur | silver molar mass | 139.93 g/mol | 32.06 g/mol | 107.8682 g/mol phase | solid (at STP) | solid (at STP) | solid (at STP) melting point | 845 °C | 112.8 °C | 960 °C boiling point | | 444.7 °C | 2212 °C density | 7.234 g/cm^3 | 2.07 g/cm^3 | 10.49 g/cm^3 solubility in water | | | insoluble
Units
