Input interpretation
silver telluride | selenium bromide
Chemical names and formulas
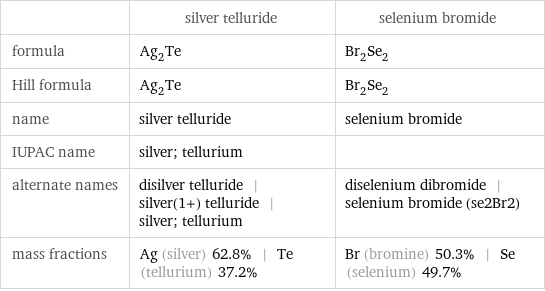
| silver telluride | selenium bromide formula | Ag_2Te | Br_2Se_2 Hill formula | Ag_2Te | Br_2Se_2 name | silver telluride | selenium bromide IUPAC name | silver; tellurium | alternate names | disilver telluride | silver(1+) telluride | silver; tellurium | diselenium dibromide | selenium bromide (se2Br2) mass fractions | Ag (silver) 62.8% | Te (tellurium) 37.2% | Br (bromine) 50.3% | Se (selenium) 49.7%
Structure diagrams

Structure diagrams
Basic properties
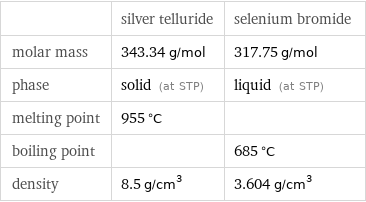
| silver telluride | selenium bromide molar mass | 343.34 g/mol | 317.75 g/mol phase | solid (at STP) | liquid (at STP) melting point | 955 °C | boiling point | | 685 °C density | 8.5 g/cm^3 | 3.604 g/cm^3
Units

Liquid properties
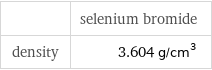
| selenium bromide density | 3.604 g/cm^3
Units

Solid properties
| silver telluride density | 8.5 g/cm^3 vapor pressure | 0.001 mmHg refractive index | 3.4
Units
