Input interpretation
gallium(III) fluoride trihydrate | lithium tetrachloroaluminate
Chemical names and formulas
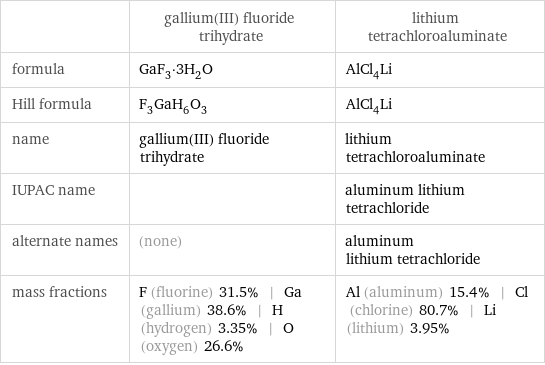
| gallium(III) fluoride trihydrate | lithium tetrachloroaluminate formula | GaF_3·3H_2O | AlCl_4Li Hill formula | F_3GaH_6O_3 | AlCl_4Li name | gallium(III) fluoride trihydrate | lithium tetrachloroaluminate IUPAC name | | aluminum lithium tetrachloride alternate names | (none) | aluminum lithium tetrachloride mass fractions | F (fluorine) 31.5% | Ga (gallium) 38.6% | H (hydrogen) 3.35% | O (oxygen) 26.6% | Al (aluminum) 15.4% | Cl (chlorine) 80.7% | Li (lithium) 3.95%
Structure diagrams
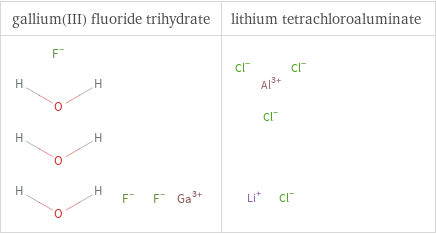
Structure diagrams
Basic properties

| gallium(III) fluoride trihydrate | lithium tetrachloroaluminate molar mass | 180.76 g/mol | 175.7 g/mol solubility in water | slightly soluble |
Units
