Input interpretation
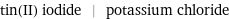
tin(II) iodide | potassium chloride
Chemical names and formulas
| tin(II) iodide | potassium chloride formula | SnI_2 | KCl Hill formula | I_2Sn | ClK name | tin(II) iodide | potassium chloride IUPAC name | diiodotin | potassium chloride alternate names | diiodotin | stannous diiodide | stannous iodide | tin diiodide | enseal | kalitabs | kaochlor | pfiklor | potavescent | rekawan mass fractions | I (iodine) 68.1% | Sn (tin) 31.9% | Cl (chlorine) 47.6% | K (potassium) 52.4%
Structure diagrams

Structure diagrams
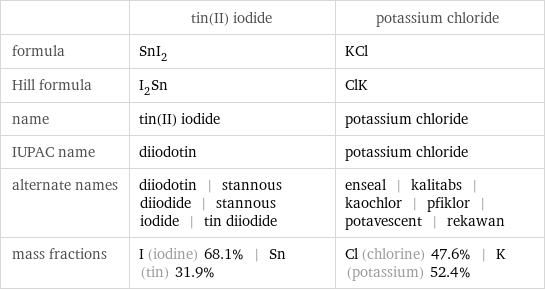
Basic properties
| tin(II) iodide | potassium chloride molar mass | 372.519 g/mol | 74.55 g/mol phase | solid (at STP) | solid (at STP) melting point | 320 °C | 770 °C boiling point | 714 °C | 1420 °C density | 5.28 g/cm^3 | 1.98 g/cm^3 solubility in water | | soluble
Units

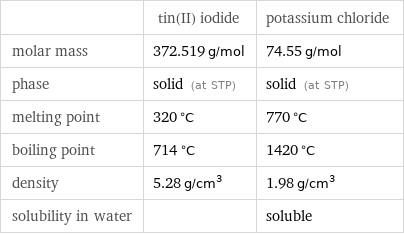
Thermodynamic properties
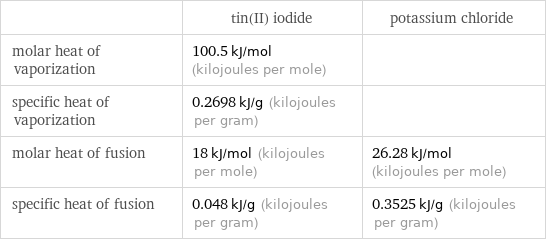
| tin(II) iodide | potassium chloride molar heat of vaporization | 100.5 kJ/mol (kilojoules per mole) | specific heat of vaporization | 0.2698 kJ/g (kilojoules per gram) | molar heat of fusion | 18 kJ/mol (kilojoules per mole) | 26.28 kJ/mol (kilojoules per mole) specific heat of fusion | 0.048 kJ/g (kilojoules per gram) | 0.3525 kJ/g (kilojoules per gram)
Solid properties (at STP)

| tin(II) iodide | potassium chloride density | 5.28 g/cm^3 | 1.98 g/cm^3 vapor pressure | 7.6 mmHg |
Units

Chemical identifiers
I | [Cl-].[K+]](../image_source/0db62d98ad30cc20e565d4bea1d3ae8d.png)
| tin(II) iodide | potassium chloride CAS number | 10294-70-9 | 7447-40-7 PubChem CID number | 25138 | 4873 PubChem SID number | | 24852090 SMILES identifier | [Sn](I)I | [Cl-].[K+]