Input interpretation
1 atom of gallium (chemical element)
Basic properties for 1 atom
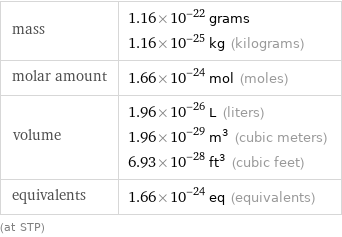
mass | 1.16×10^-22 grams 1.16×10^-25 kg (kilograms) molar amount | 1.66×10^-24 mol (moles) volume | 1.96×10^-26 L (liters) 1.96×10^-29 m^3 (cubic meters) 6.93×10^-28 ft^3 (cubic feet) equivalents | 1.66×10^-24 eq (equivalents) (at STP)
Current commodity price
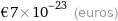
€7×10^-23 (euros)
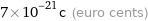
7×10^-21c (euro cents)
Thermodynamic properties for 1 atom
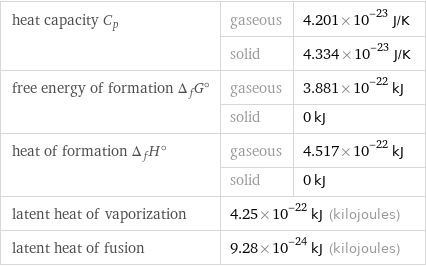
heat capacity C_p | gaseous | 4.201×10^-23 J/K | solid | 4.334×10^-23 J/K free energy of formation Δ_fG° | gaseous | 3.881×10^-22 kJ | solid | 0 kJ heat of formation Δ_fH° | gaseous | 4.517×10^-22 kJ | solid | 0 kJ latent heat of vaporization | 4.25×10^-22 kJ (kilojoules) | latent heat of fusion | 9.28×10^-24 kJ (kilojoules) |
Units
Phase change energies for 1 atom from 25 °C

energy required to heat to melting point | 2.06×10^-25 kJ (kilojoules) energy required to convert to liquid | 9.28×10^-24 kJ (kilojoules) energy required to heat to melting point and convert to liquid | 9.49×10^-24 kJ (kilojoules)
Corresponding quantities

sphere radius | 0.1673 nm (nanometers) side of a cube | 0.2697 nm (nanometers)
Structure diagram
Structure diagram
Chemical names and formulas
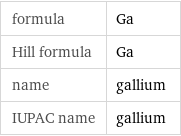
formula | Ga Hill formula | Ga name | gallium IUPAC name | gallium
Substance properties
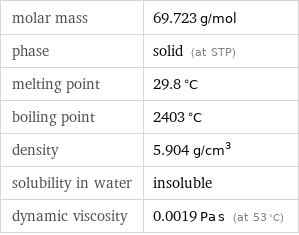
molar mass | 69.723 g/mol phase | solid (at STP) melting point | 29.8 °C boiling point | 2403 °C density | 5.904 g/cm^3 solubility in water | insoluble dynamic viscosity | 0.0019 Pa s (at 53 °C)
Units
