Input interpretation
barium fluoride | 2, 3, 5, 6-tetrafluoroaniline
Chemical names and formulas
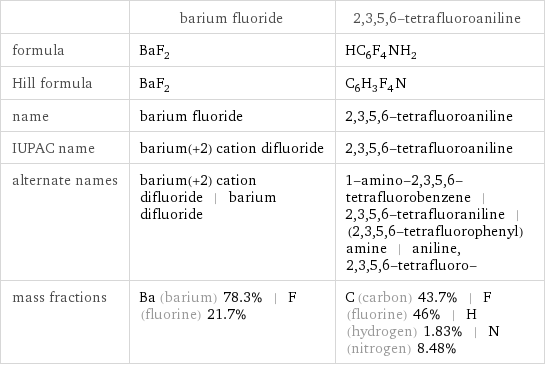
| barium fluoride | 2, 3, 5, 6-tetrafluoroaniline formula | BaF_2 | HC_6F_4NH_2 Hill formula | BaF_2 | C_6H_3F_4N name | barium fluoride | 2, 3, 5, 6-tetrafluoroaniline IUPAC name | barium(+2) cation difluoride | 2, 3, 5, 6-tetrafluoroaniline alternate names | barium(+2) cation difluoride | barium difluoride | 1-amino-2, 3, 5, 6-tetrafluorobenzene | 2, 3, 5, 6-tetrafluoraniline | (2, 3, 5, 6-tetrafluorophenyl)amine | aniline, 2, 3, 5, 6-tetrafluoro- mass fractions | Ba (barium) 78.3% | F (fluorine) 21.7% | C (carbon) 43.7% | F (fluorine) 46% | H (hydrogen) 1.83% | N (nitrogen) 8.48%
Structure diagrams
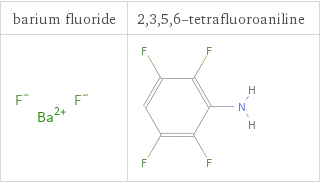
Structure diagrams
3D structure
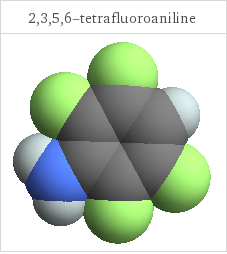
3D structure
Basic properties
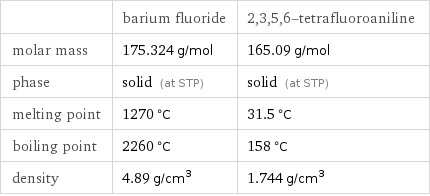
| barium fluoride | 2, 3, 5, 6-tetrafluoroaniline molar mass | 175.324 g/mol | 165.09 g/mol phase | solid (at STP) | solid (at STP) melting point | 1270 °C | 31.5 °C boiling point | 2260 °C | 158 °C density | 4.89 g/cm^3 | 1.744 g/cm^3
Units

Thermodynamic properties
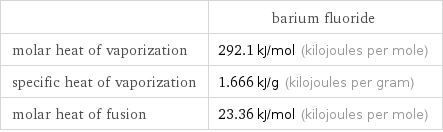
| barium fluoride molar heat of vaporization | 292.1 kJ/mol (kilojoules per mole) specific heat of vaporization | 1.666 kJ/g (kilojoules per gram) molar heat of fusion | 23.36 kJ/mol (kilojoules per mole)