Input interpretation

diprotic acids | element types
Summary
carbon | chromium | hydrogen | oxygen | phosphorus | sulfur | selenium | tellurium
Periodic table location
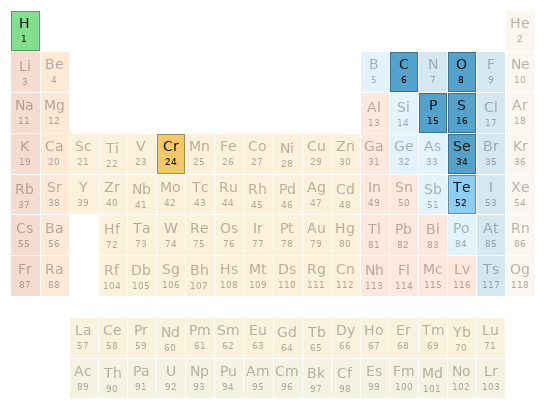
Periodic table location
Images
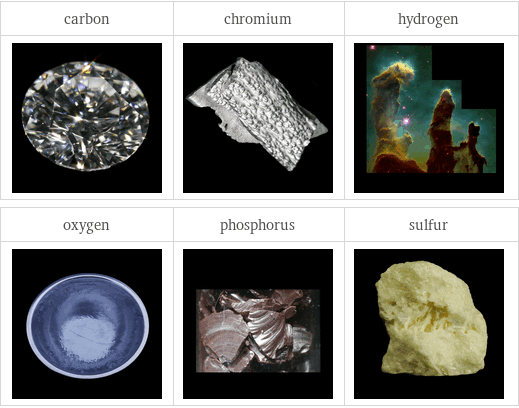
Images
Basic elemental properties
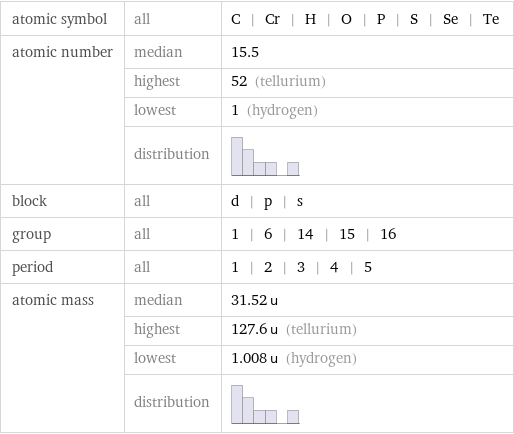
atomic symbol | all | C | Cr | H | O | P | S | Se | Te atomic number | median | 15.5 | highest | 52 (tellurium) | lowest | 1 (hydrogen) | distribution | block | all | d | p | s group | all | 1 | 6 | 14 | 15 | 16 period | all | 1 | 2 | 3 | 4 | 5 atomic mass | median | 31.52 u | highest | 127.6 u (tellurium) | lowest | 1.008 u (hydrogen) | distribution |
Table
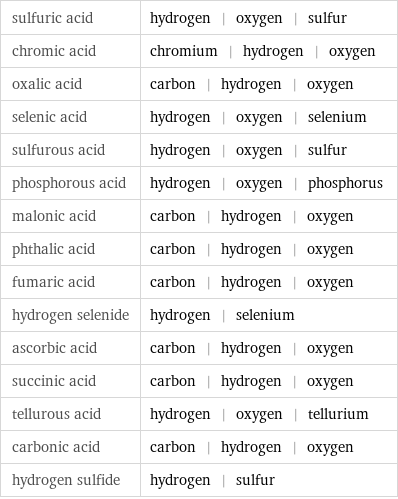
sulfuric acid | hydrogen | oxygen | sulfur chromic acid | chromium | hydrogen | oxygen oxalic acid | carbon | hydrogen | oxygen selenic acid | hydrogen | oxygen | selenium sulfurous acid | hydrogen | oxygen | sulfur phosphorous acid | hydrogen | oxygen | phosphorus malonic acid | carbon | hydrogen | oxygen phthalic acid | carbon | hydrogen | oxygen fumaric acid | carbon | hydrogen | oxygen hydrogen selenide | hydrogen | selenium ascorbic acid | carbon | hydrogen | oxygen succinic acid | carbon | hydrogen | oxygen tellurous acid | hydrogen | oxygen | tellurium carbonic acid | carbon | hydrogen | oxygen hydrogen sulfide | hydrogen | sulfur
Thermodynamic properties
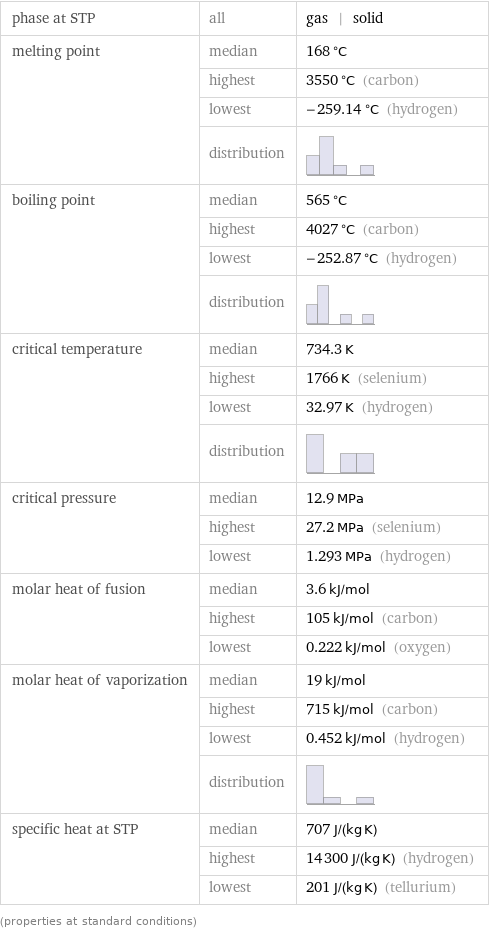
phase at STP | all | gas | solid melting point | median | 168 °C | highest | 3550 °C (carbon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | 565 °C | highest | 4027 °C (carbon) | lowest | -252.87 °C (hydrogen) | distribution | critical temperature | median | 734.3 K | highest | 1766 K (selenium) | lowest | 32.97 K (hydrogen) | distribution | critical pressure | median | 12.9 MPa | highest | 27.2 MPa (selenium) | lowest | 1.293 MPa (hydrogen) molar heat of fusion | median | 3.6 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.222 kJ/mol (oxygen) molar heat of vaporization | median | 19 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 0.452 kJ/mol (hydrogen) | distribution | specific heat at STP | median | 707 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 201 J/(kg K) (tellurium) (properties at standard conditions)
Material properties
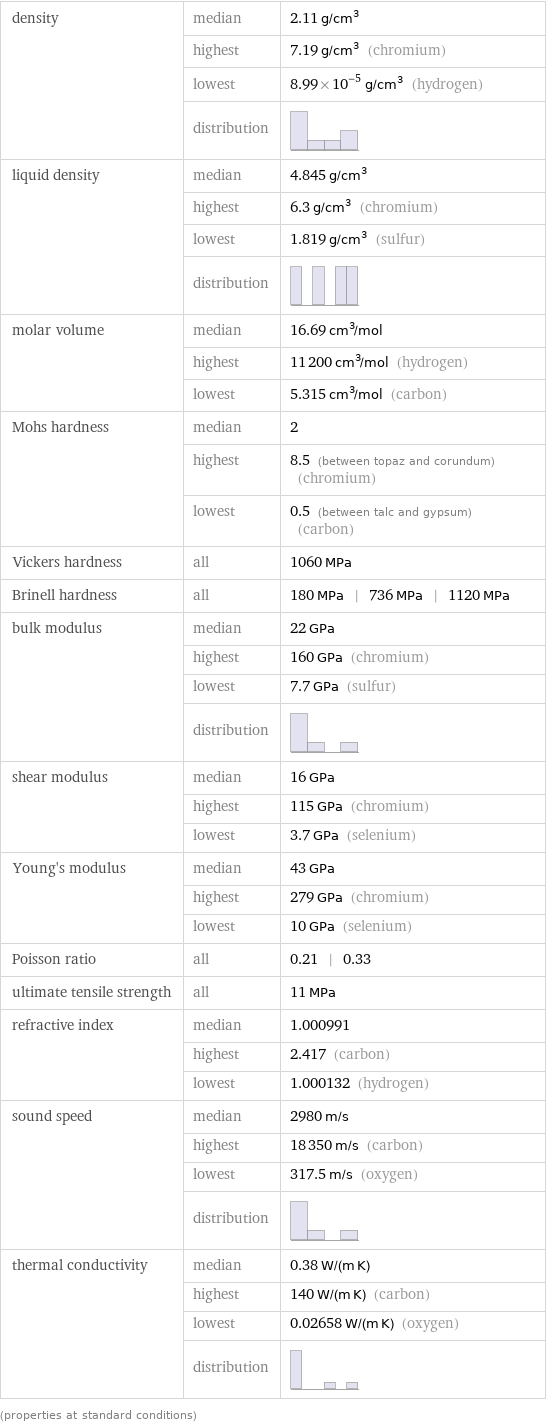
density | median | 2.11 g/cm^3 | highest | 7.19 g/cm^3 (chromium) | lowest | 8.99×10^-5 g/cm^3 (hydrogen) | distribution | liquid density | median | 4.845 g/cm^3 | highest | 6.3 g/cm^3 (chromium) | lowest | 1.819 g/cm^3 (sulfur) | distribution | molar volume | median | 16.69 cm^3/mol | highest | 11200 cm^3/mol (hydrogen) | lowest | 5.315 cm^3/mol (carbon) Mohs hardness | median | 2 | highest | 8.5 (between topaz and corundum) (chromium) | lowest | 0.5 (between talc and gypsum) (carbon) Vickers hardness | all | 1060 MPa Brinell hardness | all | 180 MPa | 736 MPa | 1120 MPa bulk modulus | median | 22 GPa | highest | 160 GPa (chromium) | lowest | 7.7 GPa (sulfur) | distribution | shear modulus | median | 16 GPa | highest | 115 GPa (chromium) | lowest | 3.7 GPa (selenium) Young's modulus | median | 43 GPa | highest | 279 GPa (chromium) | lowest | 10 GPa (selenium) Poisson ratio | all | 0.21 | 0.33 ultimate tensile strength | all | 11 MPa refractive index | median | 1.000991 | highest | 2.417 (carbon) | lowest | 1.000132 (hydrogen) sound speed | median | 2980 m/s | highest | 18350 m/s (carbon) | lowest | 317.5 m/s (oxygen) | distribution | thermal conductivity | median | 0.38 W/(m K) | highest | 140 W/(m K) (carbon) | lowest | 0.02658 W/(m K) (oxygen) | distribution | (properties at standard conditions)
Electromagnetic properties
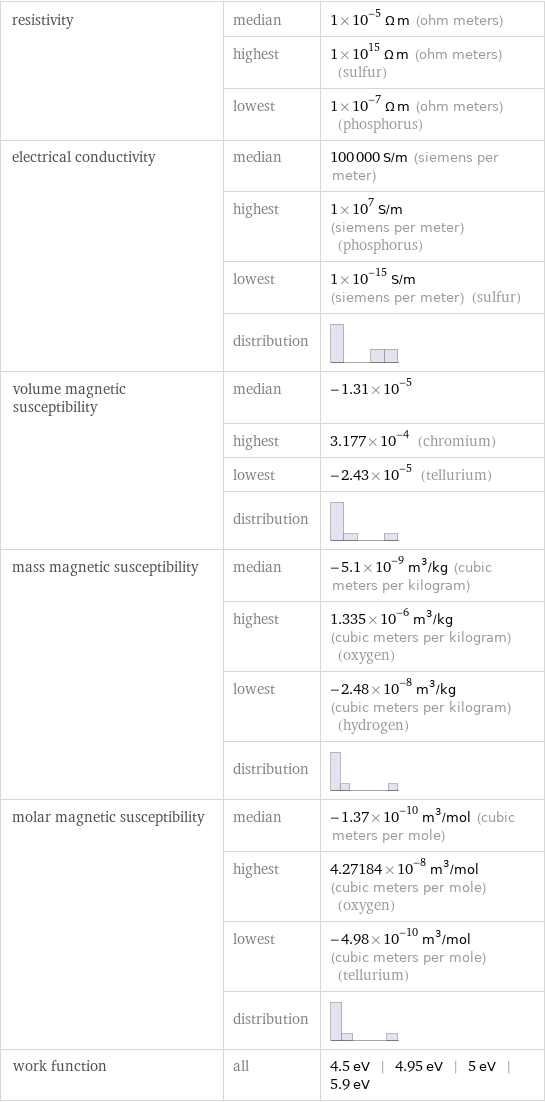
resistivity | median | 1×10^-5 Ω m (ohm meters) | highest | 1×10^15 Ω m (ohm meters) (sulfur) | lowest | 1×10^-7 Ω m (ohm meters) (phosphorus) electrical conductivity | median | 100000 S/m (siemens per meter) | highest | 1×10^7 S/m (siemens per meter) (phosphorus) | lowest | 1×10^-15 S/m (siemens per meter) (sulfur) | distribution | volume magnetic susceptibility | median | -1.31×10^-5 | highest | 3.177×10^-4 (chromium) | lowest | -2.43×10^-5 (tellurium) | distribution | mass magnetic susceptibility | median | -5.1×10^-9 m^3/kg (cubic meters per kilogram) | highest | 1.335×10^-6 m^3/kg (cubic meters per kilogram) (oxygen) | lowest | -2.48×10^-8 m^3/kg (cubic meters per kilogram) (hydrogen) | distribution | molar magnetic susceptibility | median | -1.37×10^-10 m^3/mol (cubic meters per mole) | highest | 4.27184×10^-8 m^3/mol (cubic meters per mole) (oxygen) | lowest | -4.98×10^-10 m^3/mol (cubic meters per mole) (tellurium) | distribution | work function | all | 4.5 eV | 4.95 eV | 5 eV | 5.9 eV
Reactivity
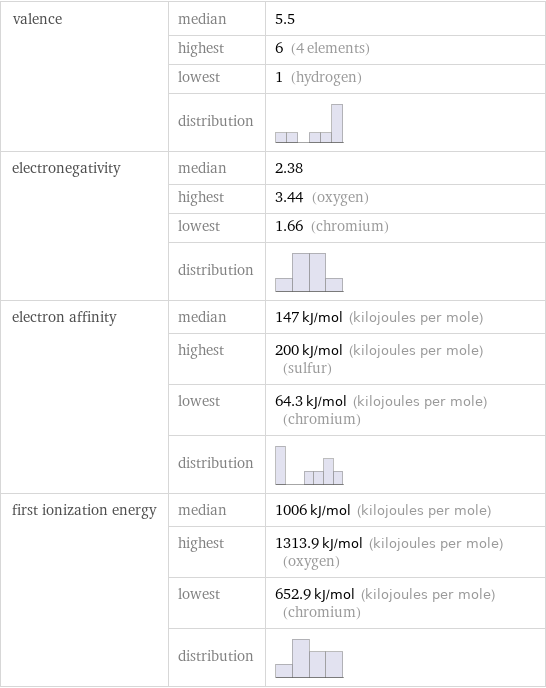
valence | median | 5.5 | highest | 6 (4 elements) | lowest | 1 (hydrogen) | distribution | electronegativity | median | 2.38 | highest | 3.44 (oxygen) | lowest | 1.66 (chromium) | distribution | electron affinity | median | 147 kJ/mol (kilojoules per mole) | highest | 200 kJ/mol (kilojoules per mole) (sulfur) | lowest | 64.3 kJ/mol (kilojoules per mole) (chromium) | distribution | first ionization energy | median | 1006 kJ/mol (kilojoules per mole) | highest | 1313.9 kJ/mol (kilojoules per mole) (oxygen) | lowest | 652.9 kJ/mol (kilojoules per mole) (chromium) | distribution |
Atomic properties
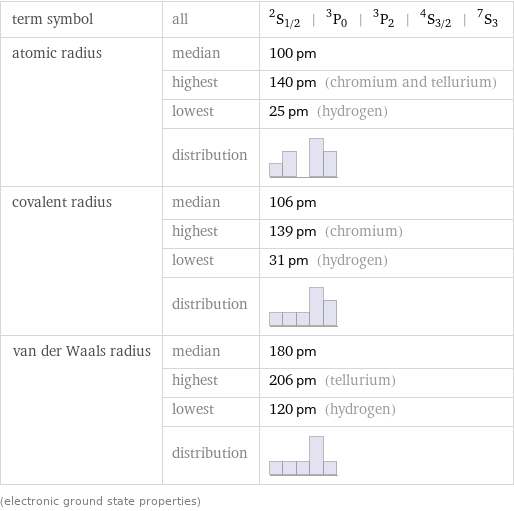
term symbol | all | ^2S_(1/2) | ^3P_0 | ^3P_2 | ^4S_(3/2) | ^7S_3 atomic radius | median | 100 pm | highest | 140 pm (chromium and tellurium) | lowest | 25 pm (hydrogen) | distribution | covalent radius | median | 106 pm | highest | 139 pm (chromium) | lowest | 31 pm (hydrogen) | distribution | van der Waals radius | median | 180 pm | highest | 206 pm (tellurium) | lowest | 120 pm (hydrogen) | distribution | (electronic ground state properties)