Input interpretation

reductants | molar mass
Summary
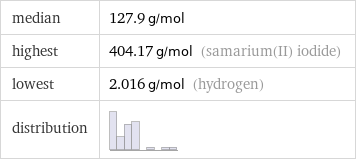
median | 127.9 g/mol highest | 404.17 g/mol (samarium(II) iodide) lowest | 2.016 g/mol (hydrogen) distribution |
Units

Distribution plots
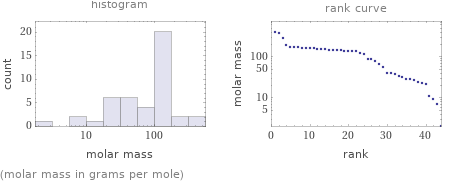
(molar mass in grams per mole)
Molar mass rankings
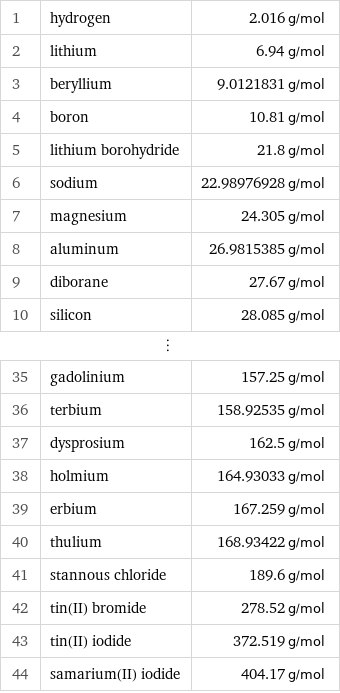
1 | hydrogen | 2.016 g/mol 2 | lithium | 6.94 g/mol 3 | beryllium | 9.0121831 g/mol 4 | boron | 10.81 g/mol 5 | lithium borohydride | 21.8 g/mol 6 | sodium | 22.98976928 g/mol 7 | magnesium | 24.305 g/mol 8 | aluminum | 26.9815385 g/mol 9 | diborane | 27.67 g/mol 10 | silicon | 28.085 g/mol ⋮ | | 35 | gadolinium | 157.25 g/mol 36 | terbium | 158.92535 g/mol 37 | dysprosium | 162.5 g/mol 38 | holmium | 164.93033 g/mol 39 | erbium | 167.259 g/mol 40 | thulium | 168.93422 g/mol 41 | stannous chloride | 189.6 g/mol 42 | tin(II) bromide | 278.52 g/mol 43 | tin(II) iodide | 372.519 g/mol 44 | samarium(II) iodide | 404.17 g/mol
Unit conversion for median molar mass 127.9 g/mol
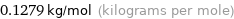
0.1279 kg/mol (kilograms per mole)
Comparisons for median molar mass 127.9 g/mol
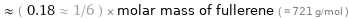
≈ ( 0.18 ≈ 1/6 ) × molar mass of fullerene ( ≈ 721 g/mol )
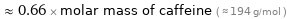
≈ 0.66 × molar mass of caffeine ( ≈ 194 g/mol )
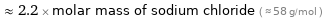
≈ 2.2 × molar mass of sodium chloride ( ≈ 58 g/mol )
Corresponding quantities
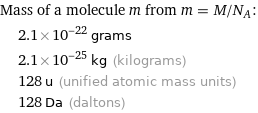
Mass of a molecule m from m = M/N_A: | 2.1×10^-22 grams | 2.1×10^-25 kg (kilograms) | 128 u (unified atomic mass units) | 128 Da (daltons)
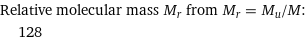
Relative molecular mass M_r from M_r = M_u/M: | 128