Input interpretation

polar protic solvents | molar heat of fusion
Summary
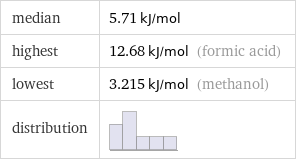
median | 5.71 kJ/mol highest | 12.68 kJ/mol (formic acid) lowest | 3.215 kJ/mol (methanol) distribution |
Units

Ranked values
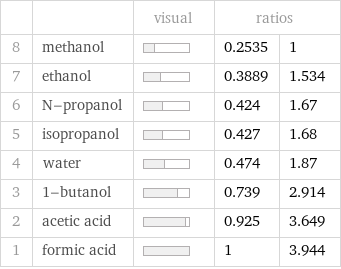
| | visual | ratios | 8 | methanol | | 0.2535 | 1 7 | ethanol | | 0.3889 | 1.534 6 | N-propanol | | 0.424 | 1.67 5 | isopropanol | | 0.427 | 1.68 4 | water | | 0.474 | 1.87 3 | 1-butanol | | 0.739 | 2.914 2 | acetic acid | | 0.925 | 3.649 1 | formic acid | | 1 | 3.944
Molar heat of fusion rankings
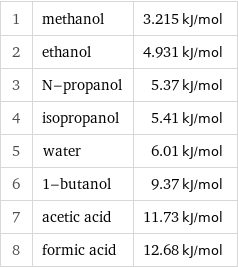
1 | methanol | 3.215 kJ/mol 2 | ethanol | 4.931 kJ/mol 3 | N-propanol | 5.37 kJ/mol 4 | isopropanol | 5.41 kJ/mol 5 | water | 6.01 kJ/mol 6 | 1-butanol | 9.37 kJ/mol 7 | acetic acid | 11.73 kJ/mol 8 | formic acid | 12.68 kJ/mol
Unit conversions for median molar heat of fusion 5.71 kJ/mol
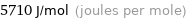
5710 J/mol (joules per mole)

1.36 kcal/mol (thermochemical kilocalories per mole)
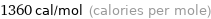
1360 cal/mol (calories per mole)

0.0592 eV (molar electronvolts)