Input interpretation
triiodide anion
Lewis structure
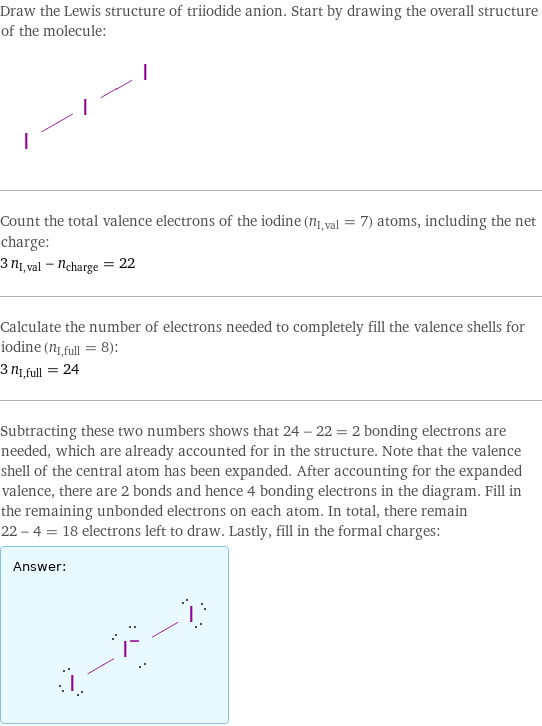
Draw the Lewis structure of triiodide anion. Start by drawing the overall structure of the molecule: Count the total valence electrons of the iodine (n_I, val = 7) atoms, including the net charge: 3 n_I, val - n_charge = 22 Calculate the number of electrons needed to completely fill the valence shells for iodine (n_I, full = 8): 3 n_I, full = 24 Subtracting these two numbers shows that 24 - 22 = 2 bonding electrons are needed, which are already accounted for in the structure. Note that the valence shell of the central atom has been expanded. After accounting for the expanded valence, there are 2 bonds and hence 4 bonding electrons in the diagram. Fill in the remaining unbonded electrons on each atom. In total, there remain 22 - 4 = 18 electrons left to draw. Lastly, fill in the formal charges: Answer: | |
General properties
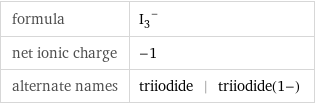
formula | (I_3)^- net ionic charge | -1 alternate names | triiodide | triiodide(1-)
Other properties

ion class | anions | ionic Lewis bases | polyhalide ions