Input interpretation
sodium iodate
Chemical names and formulas
![formula | NaIO_3 Hill formula | INaO_3 name | sodium iodate alternate names | natriumjodat [German] mass fractions | I (iodine) 64.1% | Na (sodium) 11.6% | O (oxygen) 24.3%](../image_source/a9319631416331c072408213a2ca7b8a.png)
formula | NaIO_3 Hill formula | INaO_3 name | sodium iodate alternate names | natriumjodat [German] mass fractions | I (iodine) 64.1% | Na (sodium) 11.6% | O (oxygen) 24.3%
Structure diagram
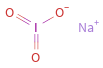
Structure diagram
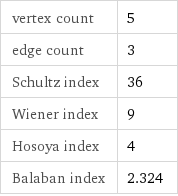
vertex count | 5 edge count | 3 Schultz index | 36 Wiener index | 9 Hosoya index | 4 Balaban index | 2.324
Basic properties
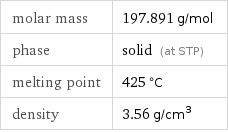
molar mass | 197.891 g/mol phase | solid (at STP) melting point | 425 °C density | 3.56 g/cm^3
Units

Solid properties (at STP)

density | 3.56 g/cm^3
Units

Thermodynamic properties
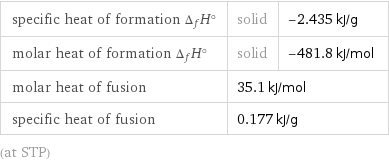
specific heat of formation Δ_fH° | solid | -2.435 kJ/g molar heat of formation Δ_fH° | solid | -481.8 kJ/mol molar heat of fusion | 35.1 kJ/mol | specific heat of fusion | 0.177 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7681-55-2 PubChem CID number | 24344 SMILES identifier | [O-]I(=O)=O.[Na+] InChI identifier | InChI=1/HIO3.Na/c2-1(3)4;/h(H, 2, 3, 4);/q;+1/p-1/fIO3.Na/q-1;m EU number | 231-672-5 Gmelin number | 8539 RTECS number | NN1400000 NSC number | 77387](../image_source/a8e361348c9418992a98d6896d9854dc.png)
CAS number | 7681-55-2 PubChem CID number | 24344 SMILES identifier | [O-]I(=O)=O.[Na+] InChI identifier | InChI=1/HIO3.Na/c2-1(3)4;/h(H, 2, 3, 4);/q;+1/p-1/fIO3.Na/q-1;m EU number | 231-672-5 Gmelin number | 8539 RTECS number | NN1400000 NSC number | 77387
Toxicity properties

RTECS classes | other
Ion equivalents
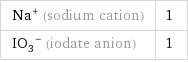
Na^+ (sodium cation) | 1 (IO_3)^- (iodate anion) | 1