Input interpretation

polar protic solvents | proton count
Summary
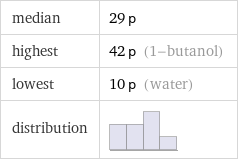
median | 29 p highest | 42 p (1-butanol) lowest | 10 p (water) distribution |
Units

Ranked values
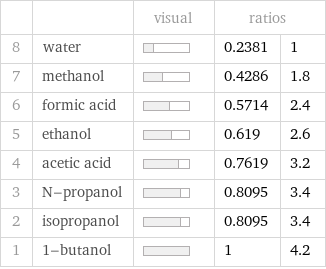
| | visual | ratios | 8 | water | | 0.2381 | 1 7 | methanol | | 0.4286 | 1.8 6 | formic acid | | 0.5714 | 2.4 5 | ethanol | | 0.619 | 2.6 4 | acetic acid | | 0.7619 | 3.2 3 | N-propanol | | 0.8095 | 3.4 2 | isopropanol | | 0.8095 | 3.4 1 | 1-butanol | | 1 | 4.2
Proton count rankings
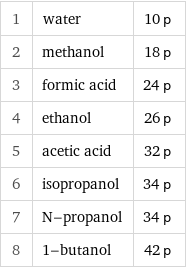
1 | water | 10 p 2 | methanol | 18 p 3 | formic acid | 24 p 4 | ethanol | 26 p 5 | acetic acid | 32 p 6 | isopropanol | 34 p 7 | N-propanol | 34 p 8 | 1-butanol | 42 p
Corresponding quantity
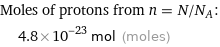
Moles of protons from n = N/N_A: | 4.8×10^-23 mol (moles)