Input interpretation
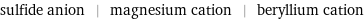
sulfide anion | magnesium cation | beryllium cation
Structure diagrams
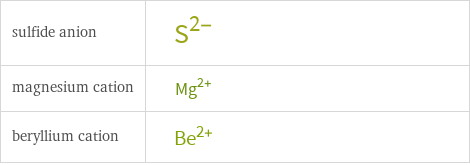
Structure diagrams
General properties
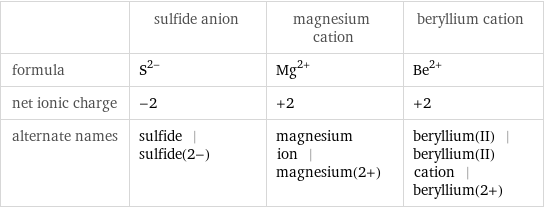
| sulfide anion | magnesium cation | beryllium cation formula | S^(2-) | Mg^(2+) | Be^(2+) net ionic charge | -2 | +2 | +2 alternate names | sulfide | sulfide(2-) | magnesium ion | magnesium(2+) | beryllium(II) | beryllium(II) cation | beryllium(2+)
Ionic radii
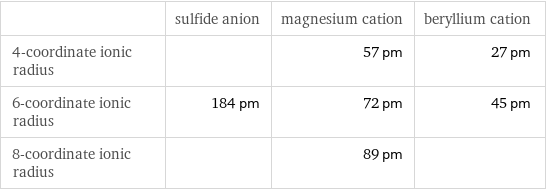
| sulfide anion | magnesium cation | beryllium cation 4-coordinate ionic radius | | 57 pm | 27 pm 6-coordinate ionic radius | 184 pm | 72 pm | 45 pm 8-coordinate ionic radius | | 89 pm |
Units

Other properties
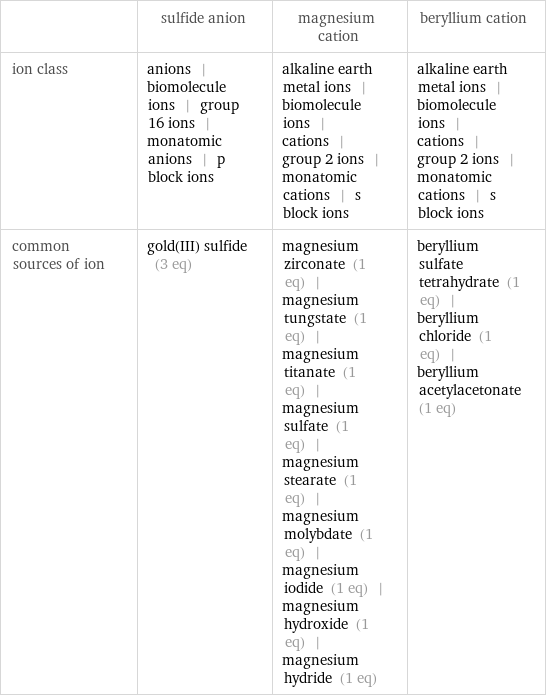
| sulfide anion | magnesium cation | beryllium cation ion class | anions | biomolecule ions | group 16 ions | monatomic anions | p block ions | alkaline earth metal ions | biomolecule ions | cations | group 2 ions | monatomic cations | s block ions | alkaline earth metal ions | biomolecule ions | cations | group 2 ions | monatomic cations | s block ions common sources of ion | gold(III) sulfide (3 eq) | magnesium zirconate (1 eq) | magnesium tungstate (1 eq) | magnesium titanate (1 eq) | magnesium sulfate (1 eq) | magnesium stearate (1 eq) | magnesium molybdate (1 eq) | magnesium iodide (1 eq) | magnesium hydroxide (1 eq) | magnesium hydride (1 eq) | beryllium sulfate tetrahydrate (1 eq) | beryllium chloride (1 eq) | beryllium acetylacetonate (1 eq)