Input interpretation
scandium chloride | calcium bisulfite
Chemical names and formulas
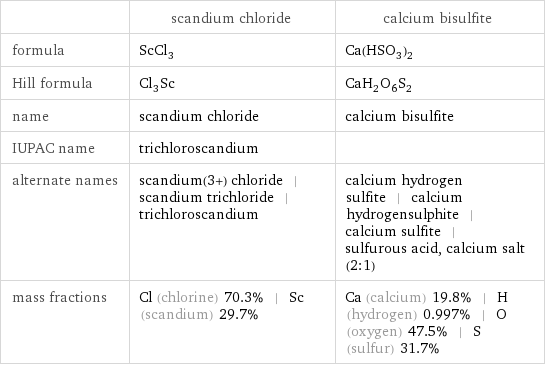
| scandium chloride | calcium bisulfite formula | ScCl_3 | Ca(HSO_3)_2 Hill formula | Cl_3Sc | CaH_2O_6S_2 name | scandium chloride | calcium bisulfite IUPAC name | trichloroscandium | alternate names | scandium(3+) chloride | scandium trichloride | trichloroscandium | calcium hydrogen sulfite | calcium hydrogensulphite | calcium sulfite | sulfurous acid, calcium salt (2:1) mass fractions | Cl (chlorine) 70.3% | Sc (scandium) 29.7% | Ca (calcium) 19.8% | H (hydrogen) 0.997% | O (oxygen) 47.5% | S (sulfur) 31.7%
Structure diagrams
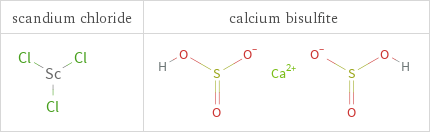
Structure diagrams
3D structure
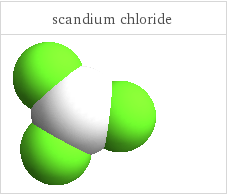
3D structure
Basic properties
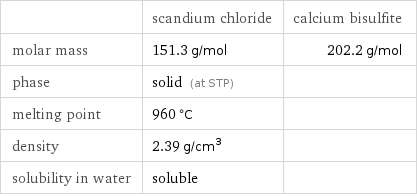
| scandium chloride | calcium bisulfite molar mass | 151.3 g/mol | 202.2 g/mol phase | solid (at STP) | melting point | 960 °C | density | 2.39 g/cm^3 | solubility in water | soluble |
Units
