Input interpretation
sodium chlorate | samarium(III) chloride
Chemical names and formulas
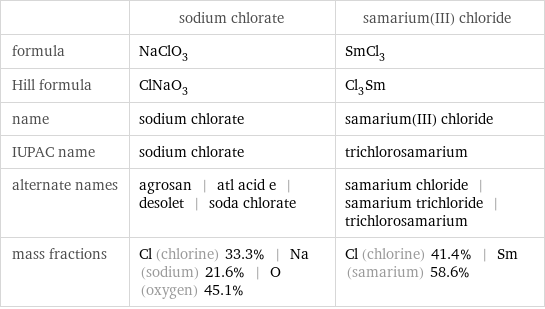
| sodium chlorate | samarium(III) chloride formula | NaClO_3 | SmCl_3 Hill formula | ClNaO_3 | Cl_3Sm name | sodium chlorate | samarium(III) chloride IUPAC name | sodium chlorate | trichlorosamarium alternate names | agrosan | atl acid e | desolet | soda chlorate | samarium chloride | samarium trichloride | trichlorosamarium mass fractions | Cl (chlorine) 33.3% | Na (sodium) 21.6% | O (oxygen) 45.1% | Cl (chlorine) 41.4% | Sm (samarium) 58.6%
Structure diagrams
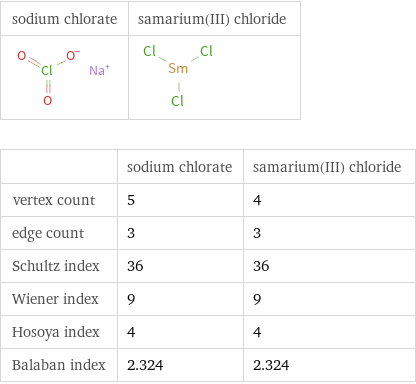
| sodium chlorate | samarium(III) chloride vertex count | 5 | 4 edge count | 3 | 3 Schultz index | 36 | 36 Wiener index | 9 | 9 Hosoya index | 4 | 4 Balaban index | 2.324 | 2.324
3D structure
3D structure
Basic properties
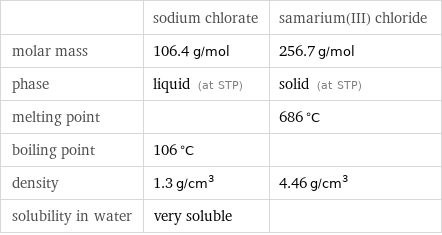
| sodium chlorate | samarium(III) chloride molar mass | 106.4 g/mol | 256.7 g/mol phase | liquid (at STP) | solid (at STP) melting point | | 686 °C boiling point | 106 °C | density | 1.3 g/cm^3 | 4.46 g/cm^3 solubility in water | very soluble |
Units

Liquid properties
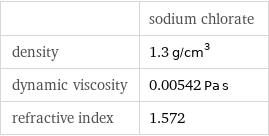
| sodium chlorate density | 1.3 g/cm^3 dynamic viscosity | 0.00542 Pa s refractive index | 1.572
Units

Thermodynamic properties
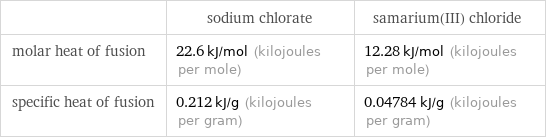
| sodium chlorate | samarium(III) chloride molar heat of fusion | 22.6 kJ/mol (kilojoules per mole) | 12.28 kJ/mol (kilojoules per mole) specific heat of fusion | 0.212 kJ/g (kilojoules per gram) | 0.04784 kJ/g (kilojoules per gram)
Solid properties

| samarium(III) chloride density | 4.46 g/cm^3 vapor pressure | 1 mmHg
Units
