Input interpretation
nitrosyl chloride | nitrogen trifluoride
Chemical names and formulas
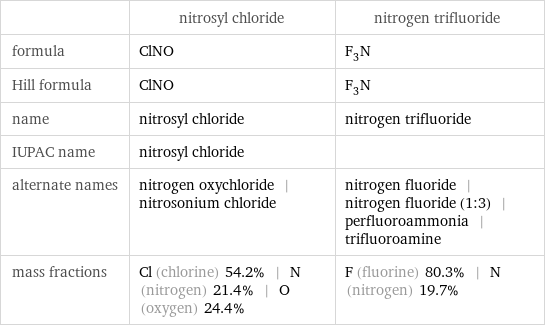
| nitrosyl chloride | nitrogen trifluoride formula | ClNO | F_3N Hill formula | ClNO | F_3N name | nitrosyl chloride | nitrogen trifluoride IUPAC name | nitrosyl chloride | alternate names | nitrogen oxychloride | nitrosonium chloride | nitrogen fluoride | nitrogen fluoride (1:3) | perfluoroammonia | trifluoroamine mass fractions | Cl (chlorine) 54.2% | N (nitrogen) 21.4% | O (oxygen) 24.4% | F (fluorine) 80.3% | N (nitrogen) 19.7%
Structure diagrams
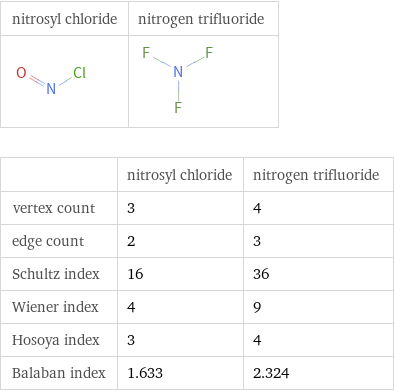
| nitrosyl chloride | nitrogen trifluoride vertex count | 3 | 4 edge count | 2 | 3 Schultz index | 16 | 36 Wiener index | 4 | 9 Hosoya index | 3 | 4 Balaban index | 1.633 | 2.324
3D structure
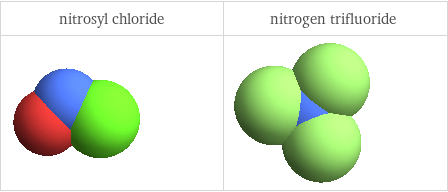
3D structure
Basic properties
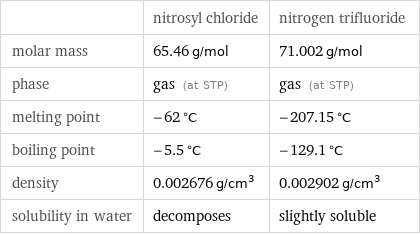
| nitrosyl chloride | nitrogen trifluoride molar mass | 65.46 g/mol | 71.002 g/mol phase | gas (at STP) | gas (at STP) melting point | -62 °C | -207.15 °C boiling point | -5.5 °C | -129.1 °C density | 0.002676 g/cm^3 | 0.002902 g/cm^3 solubility in water | decomposes | slightly soluble
Units

Gas properties (at STP)
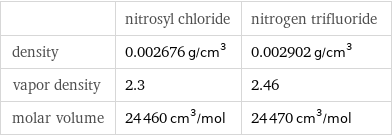
| nitrosyl chloride | nitrogen trifluoride density | 0.002676 g/cm^3 | 0.002902 g/cm^3 vapor density | 2.3 | 2.46 molar volume | 24460 cm^3/mol | 24470 cm^3/mol
Units

Thermodynamic properties
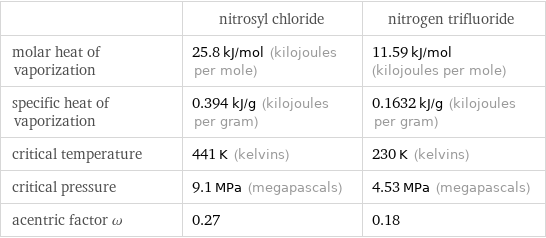
| nitrosyl chloride | nitrogen trifluoride molar heat of vaporization | 25.8 kJ/mol (kilojoules per mole) | 11.59 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.394 kJ/g (kilojoules per gram) | 0.1632 kJ/g (kilojoules per gram) critical temperature | 441 K (kelvins) | 230 K (kelvins) critical pressure | 9.1 MPa (megapascals) | 4.53 MPa (megapascals) acentric factor ω | 0.27 | 0.18
Chemical identifiers

| nitrosyl chloride | nitrogen trifluoride CAS number | 2696-92-6 | 7783-54-2 PubChem CID number | 17601 | 24553 SMILES identifier | N(=O)Cl | N(F)(F)F