Input interpretation
ammonium azide | zinc dichromate
Chemical names and formulas
![| ammonium azide | zinc dichromate formula | H_4N_4 | ZnCr_2O_7 Hill formula | H_4N_4 | Cr_2O_7Zn name | ammonium azide | zinc dichromate IUPAC name | azanium;azide | zinc oxido-(oxido-dioxo-chromio)oxy-dioxo-chromium alternate names | ammonium azide [Forbidden] | chromic acid, zinc salt (1:1) | dichromic acid, zinc salt (1:1) | zinc bichromate | zinc chromium oxide mass fractions | H (hydrogen) 6.71% | N (nitrogen) 93.3% | Cr (chromium) 37% | O (oxygen) 39.8% | Zn (zinc) 23.2%](../image_source/2b495f12d7a3e75de85baf2ae21dc29d.png)
| ammonium azide | zinc dichromate formula | H_4N_4 | ZnCr_2O_7 Hill formula | H_4N_4 | Cr_2O_7Zn name | ammonium azide | zinc dichromate IUPAC name | azanium;azide | zinc oxido-(oxido-dioxo-chromio)oxy-dioxo-chromium alternate names | ammonium azide [Forbidden] | chromic acid, zinc salt (1:1) | dichromic acid, zinc salt (1:1) | zinc bichromate | zinc chromium oxide mass fractions | H (hydrogen) 6.71% | N (nitrogen) 93.3% | Cr (chromium) 37% | O (oxygen) 39.8% | Zn (zinc) 23.2%
Structure diagrams
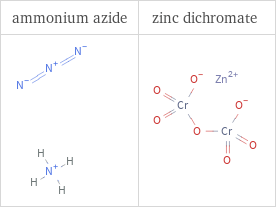
Structure diagrams
Basic properties
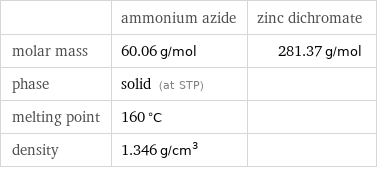
| ammonium azide | zinc dichromate molar mass | 60.06 g/mol | 281.37 g/mol phase | solid (at STP) | melting point | 160 °C | density | 1.346 g/cm^3 |
Units
