Input interpretation

potassium pyrosulfate | rhenium(VI) oxytetrafluoride
Chemical names and formulas
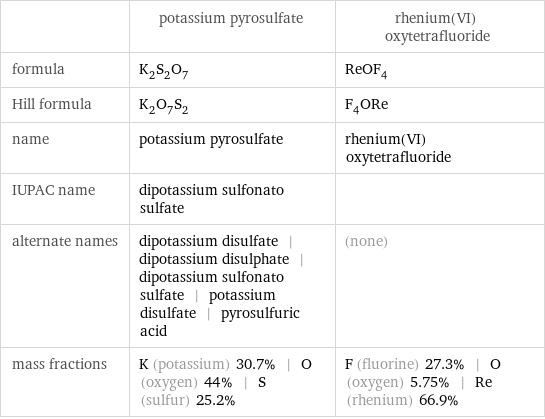
| potassium pyrosulfate | rhenium(VI) oxytetrafluoride formula | K_2S_2O_7 | ReOF_4 Hill formula | K_2O_7S_2 | F_4ORe name | potassium pyrosulfate | rhenium(VI) oxytetrafluoride IUPAC name | dipotassium sulfonato sulfate | alternate names | dipotassium disulfate | dipotassium disulphate | dipotassium sulfonato sulfate | potassium disulfate | pyrosulfuric acid | (none) mass fractions | K (potassium) 30.7% | O (oxygen) 44% | S (sulfur) 25.2% | F (fluorine) 27.3% | O (oxygen) 5.75% | Re (rhenium) 66.9%
Structure diagrams
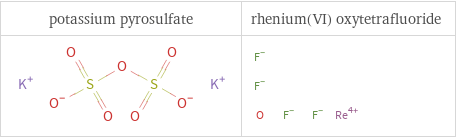
Structure diagrams
Basic properties
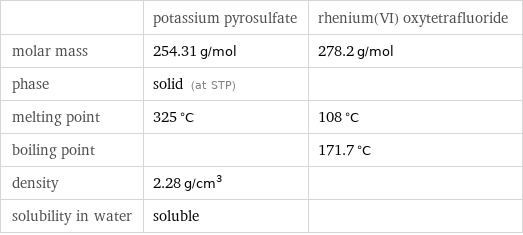
| potassium pyrosulfate | rhenium(VI) oxytetrafluoride molar mass | 254.31 g/mol | 278.2 g/mol phase | solid (at STP) | melting point | 325 °C | 108 °C boiling point | | 171.7 °C density | 2.28 g/cm^3 | solubility in water | soluble |
Units

Solid properties

| potassium pyrosulfate density | 2.28 g/cm^3
Units
