Input interpretation
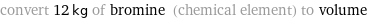
convert 12 kg of bromine (chemical element) to volume
Results
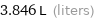
3.846 L (liters)
Possible intermediate steps
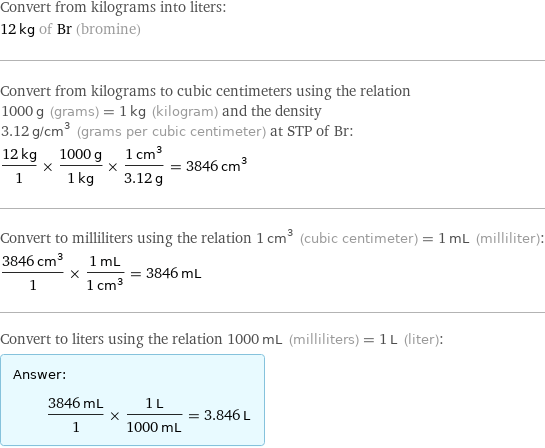
Convert from kilograms into liters: 12 kg of Br (bromine) Convert from kilograms to cubic centimeters using the relation 1000 g (grams) = 1 kg (kilogram) and the density 3.12 g/cm^3 (grams per cubic centimeter) at STP of Br: (12 kg)/1 × (1000 g)/(1 kg) × (1 cm^3)/(3.12 g) = 3846 cm^3 Convert to milliliters using the relation 1 cm^3 (cubic centimeter) = 1 mL (milliliter): (3846 cm^3)/1 × (1 mL)/(1 cm^3) = 3846 mL Convert to liters using the relation 1000 mL (milliliters) = 1 L (liter): Answer: | | (3846 mL)/1 × (1 L)/(1000 mL) = 3.846 L
Unit conversions
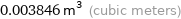
0.003846 m^3 (cubic meters)
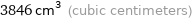
3846 cm^3 (cubic centimeters)
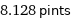
8.128 pints
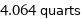
4.064 quarts
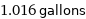
1.016 gallons
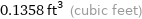
0.1358 ft^3 (cubic feet)
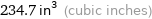
234.7 in^3 (cubic inches)
Comparisons as volume
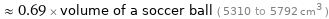
≈ 0.69 × volume of a soccer ball ( 5310 to 5792 cm^3 )
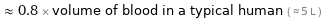
≈ 0.8 × volume of blood in a typical human ( ≈ 5 L )
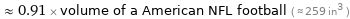
≈ 0.91 × volume of a American NFL football ( ≈ 259 in^3 )
Comparisons as volume of food or beverage
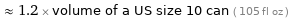
≈ 1.2 × volume of a US size 10 can ( 105 fl oz )
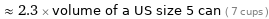
≈ 2.3 × volume of a US size 5 can ( 7 cups )
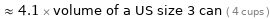
≈ 4.1 × volume of a US size 3 can ( 4 cups )
Interpretations
volume
volume of food or beverage
capacity
section modulus
Basic unit dimensions
![[length]^3](../image_source/044c0f397606e62a5cfc80ce92b45ab5.png)
[length]^3
Corresponding quantities
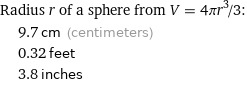
Radius r of a sphere from V = 4πr^3/3: | 9.7 cm (centimeters) | 0.32 feet | 3.8 inches
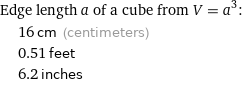
Edge length a of a cube from V = a^3: | 16 cm (centimeters) | 0.51 feet | 6.2 inches
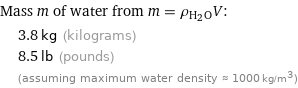
Mass m of water from m = ρ_(H_2O)V: | 3.8 kg (kilograms) | 8.5 lb (pounds) | (assuming maximum water density ≈ 1000 kg/m^3)
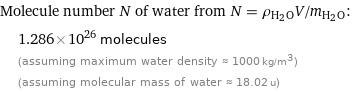
Molecule number N of water from N = ρ_(H_2O)V/m_(H_2O): | 1.286×10^26 molecules | (assuming maximum water density ≈ 1000 kg/m^3) | (assuming molecular mass of water ≈ 18.02 u)