Input interpretation

alkyl halides | Henry law constant
Summary

median | 893.7 Pa m^3/mol highest | 465082 Pa m^3/mol (tetrafluoromethane) lowest | 102.3 Pa m^3/mol (fluoroform) | (based on 5 values; 15 unavailable)
Entities with missing values
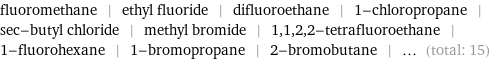
fluoromethane | ethyl fluoride | difluoroethane | 1-chloropropane | sec-butyl chloride | methyl bromide | 1, 1, 2, 2-tetrafluoroethane | 1-fluorohexane | 1-bromopropane | 2-bromobutane | ... (total: 15)
Henry law constant rankings
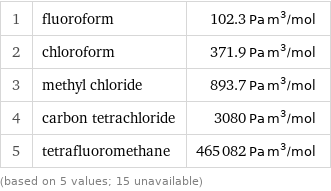
1 | fluoroform | 102.3 Pa m^3/mol 2 | chloroform | 371.9 Pa m^3/mol 3 | methyl chloride | 893.7 Pa m^3/mol 4 | carbon tetrachloride | 3080 Pa m^3/mol 5 | tetrafluoromethane | 465082 Pa m^3/mol (based on 5 values; 15 unavailable)
Unit conversions for median Henry law constant 893.7 Pa m^3/mol
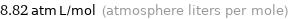
8.82 atm L/mol (atmosphere liters per mole)

0.00882 atm m^3/mol (atmosphere cubic meters per mole)