Input interpretation
disodium hydrogen phosphate
Chemical names and formulas
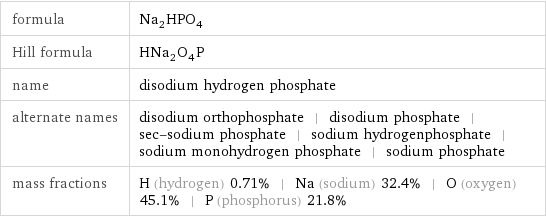
formula | Na_2HPO_4 Hill formula | HNa_2O_4P name | disodium hydrogen phosphate alternate names | disodium orthophosphate | disodium phosphate | sec-sodium phosphate | sodium hydrogenphosphate | sodium monohydrogen phosphate | sodium phosphate mass fractions | H (hydrogen) 0.71% | Na (sodium) 32.4% | O (oxygen) 45.1% | P (phosphorus) 21.8%
Structure diagram
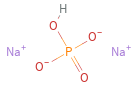
Structure diagram
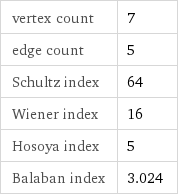
vertex count | 7 edge count | 5 Schultz index | 64 Wiener index | 16 Hosoya index | 5 Balaban index | 3.024
Basic properties
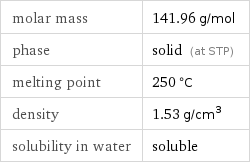
molar mass | 141.96 g/mol phase | solid (at STP) melting point | 250 °C density | 1.53 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)

density | 1.53 g/cm^3
Units

Thermodynamic properties
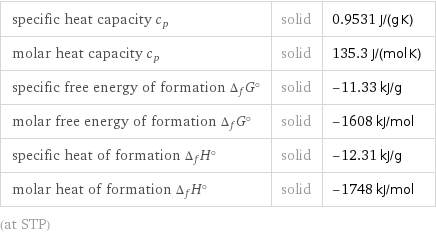
specific heat capacity c_p | solid | 0.9531 J/(g K) molar heat capacity c_p | solid | 135.3 J/(mol K) specific free energy of formation Δ_fG° | solid | -11.33 kJ/g molar free energy of formation Δ_fG° | solid | -1608 kJ/mol specific heat of formation Δ_fH° | solid | -12.31 kJ/g molar heat of formation Δ_fH° | solid | -1748 kJ/mol (at STP)
Chemical identifiers
![CAS number | 7558-79-4 PubChem CID number | 24203 SMILES identifier | OP(=O)([O-])[O-].[Na+].[Na+] InChI identifier | InChI=1/2Na.H3O4P/c;;1-5(2, 3)4/h;;(H3, 1, 2, 3, 4)/q2*+1;/p-2/f2Na.HO4P/h;;1H/q2m;-2 RTECS number | WC4500000 MDL number | MFCD00003496](../image_source/9f9c372498c4ab5a4c2d8fec3622d3e4.png)
CAS number | 7558-79-4 PubChem CID number | 24203 SMILES identifier | OP(=O)([O-])[O-].[Na+].[Na+] InChI identifier | InChI=1/2Na.H3O4P/c;;1-5(2, 3)4/h;;(H3, 1, 2, 3, 4)/q2*+1;/p-2/f2Na.HO4P/h;;1H/q2m;-2 RTECS number | WC4500000 MDL number | MFCD00003496
NFPA label

NFPA label

NFPA health rating | 2 NFPA fire rating | 0 NFPA reactivity rating | 0
Toxicity properties

RTECS classes | primary irritant
Ion equivalents
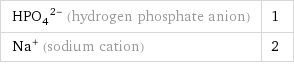
(HPO_4)^(2-) (hydrogen phosphate anion) | 1 Na^+ (sodium cation) | 2