Input interpretation
rubidium fluoride | zinc fluoride
Chemical names and formulas
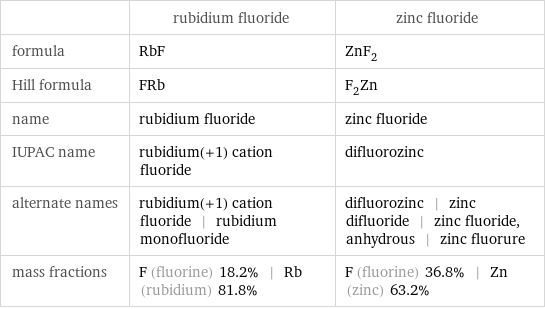
| rubidium fluoride | zinc fluoride formula | RbF | ZnF_2 Hill formula | FRb | F_2Zn name | rubidium fluoride | zinc fluoride IUPAC name | rubidium(+1) cation fluoride | difluorozinc alternate names | rubidium(+1) cation fluoride | rubidium monofluoride | difluorozinc | zinc difluoride | zinc fluoride, anhydrous | zinc fluorure mass fractions | F (fluorine) 18.2% | Rb (rubidium) 81.8% | F (fluorine) 36.8% | Zn (zinc) 63.2%
Structure diagrams

Structure diagrams
Basic properties
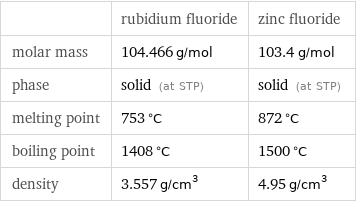
| rubidium fluoride | zinc fluoride molar mass | 104.466 g/mol | 103.4 g/mol phase | solid (at STP) | solid (at STP) melting point | 753 °C | 872 °C boiling point | 1408 °C | 1500 °C density | 3.557 g/cm^3 | 4.95 g/cm^3
Units

Thermodynamic properties
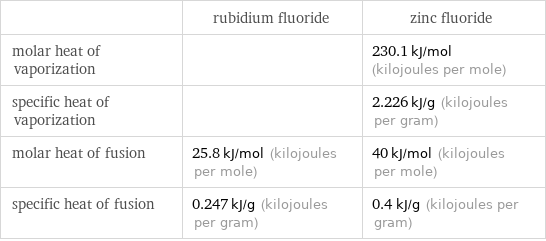
| rubidium fluoride | zinc fluoride molar heat of vaporization | | 230.1 kJ/mol (kilojoules per mole) specific heat of vaporization | | 2.226 kJ/g (kilojoules per gram) molar heat of fusion | 25.8 kJ/mol (kilojoules per mole) | 40 kJ/mol (kilojoules per mole) specific heat of fusion | 0.247 kJ/g (kilojoules per gram) | 0.4 kJ/g (kilojoules per gram)
Solid properties (at STP)

| rubidium fluoride | zinc fluoride density | 3.557 g/cm^3 | 4.95 g/cm^3 vapor pressure | 98.9 mmHg | 0.9998 mmHg
Units
