Input interpretation
chlorine trifluoride | hydrogen
Chemical names and formulas
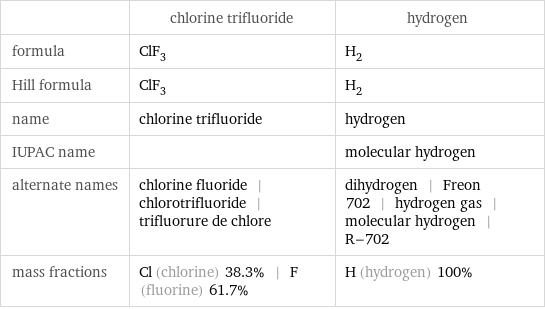
| chlorine trifluoride | hydrogen formula | ClF_3 | H_2 Hill formula | ClF_3 | H_2 name | chlorine trifluoride | hydrogen IUPAC name | | molecular hydrogen alternate names | chlorine fluoride | chlorotrifluoride | trifluorure de chlore | dihydrogen | Freon 702 | hydrogen gas | molecular hydrogen | R-702 mass fractions | Cl (chlorine) 38.3% | F (fluorine) 61.7% | H (hydrogen) 100%
Structure diagrams
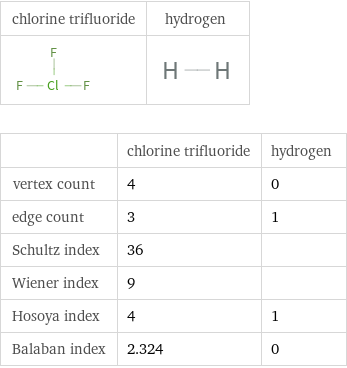
| chlorine trifluoride | hydrogen vertex count | 4 | 0 edge count | 3 | 1 Schultz index | 36 | Wiener index | 9 | Hosoya index | 4 | 1 Balaban index | 2.324 | 0
3D structure
3D structure
Basic properties
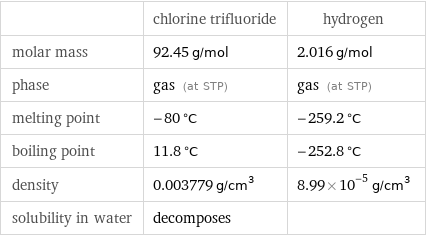
| chlorine trifluoride | hydrogen molar mass | 92.45 g/mol | 2.016 g/mol phase | gas (at STP) | gas (at STP) melting point | -80 °C | -259.2 °C boiling point | 11.8 °C | -252.8 °C density | 0.003779 g/cm^3 | 8.99×10^-5 g/cm^3 solubility in water | decomposes |
Units

Gas properties (at STP)
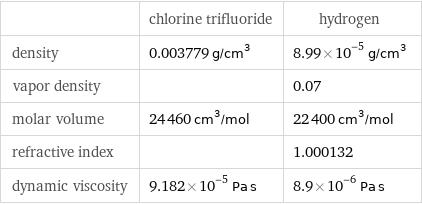
| chlorine trifluoride | hydrogen density | 0.003779 g/cm^3 | 8.99×10^-5 g/cm^3 vapor density | | 0.07 molar volume | 24460 cm^3/mol | 22400 cm^3/mol refractive index | | 1.000132 dynamic viscosity | 9.182×10^-5 Pa s | 8.9×10^-6 Pa s
Units
Thermodynamic properties
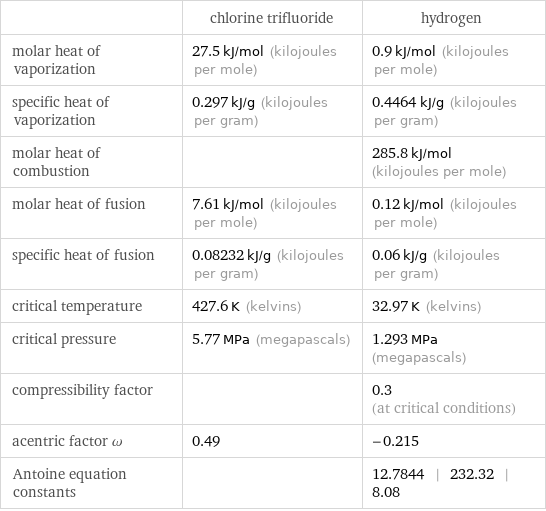
| chlorine trifluoride | hydrogen molar heat of vaporization | 27.5 kJ/mol (kilojoules per mole) | 0.9 kJ/mol (kilojoules per mole) specific heat of vaporization | 0.297 kJ/g (kilojoules per gram) | 0.4464 kJ/g (kilojoules per gram) molar heat of combustion | | 285.8 kJ/mol (kilojoules per mole) molar heat of fusion | 7.61 kJ/mol (kilojoules per mole) | 0.12 kJ/mol (kilojoules per mole) specific heat of fusion | 0.08232 kJ/g (kilojoules per gram) | 0.06 kJ/g (kilojoules per gram) critical temperature | 427.6 K (kelvins) | 32.97 K (kelvins) critical pressure | 5.77 MPa (megapascals) | 1.293 MPa (megapascals) compressibility factor | | 0.3 (at critical conditions) acentric factor ω | 0.49 | -0.215 Antoine equation constants | | 12.7844 | 232.32 | 8.08