Input interpretation
periodic acid
Chemical names and formulas

formula | H_5IO_6 name | periodic acid alternate names | orthoperiodic acid | paraperiodic acid mass fractions | H (hydrogen) 2.21% | I (iodine) 55.7% | O (oxygen) 42.1%
Lewis structure
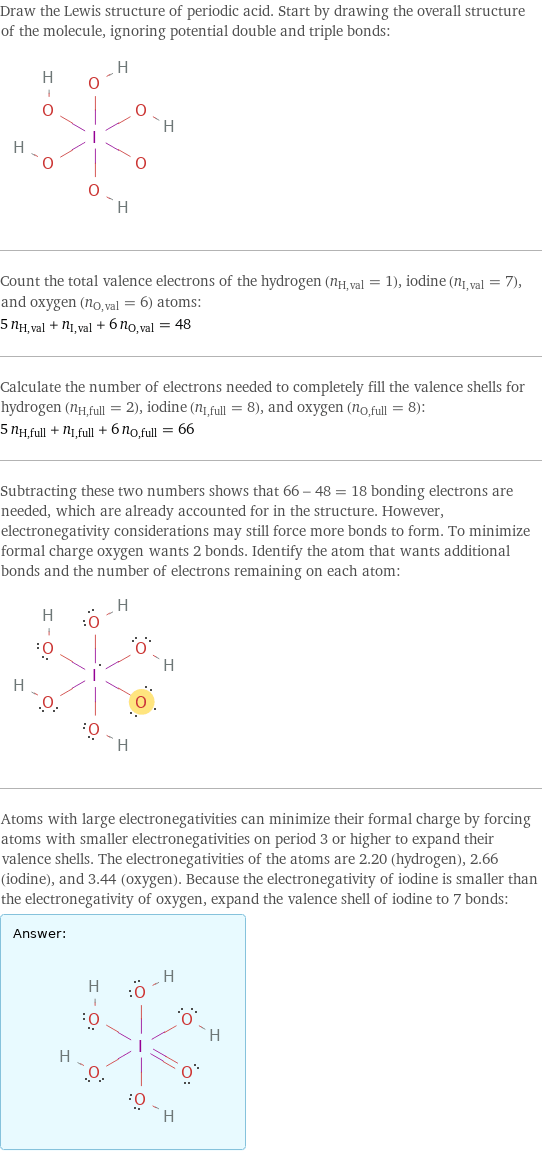
Draw the Lewis structure of periodic acid. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the hydrogen (n_H, val = 1), iodine (n_I, val = 7), and oxygen (n_O, val = 6) atoms: 5 n_H, val + n_I, val + 6 n_O, val = 48 Calculate the number of electrons needed to completely fill the valence shells for hydrogen (n_H, full = 2), iodine (n_I, full = 8), and oxygen (n_O, full = 8): 5 n_H, full + n_I, full + 6 n_O, full = 66 Subtracting these two numbers shows that 66 - 48 = 18 bonding electrons are needed, which are already accounted for in the structure. However, electronegativity considerations may still force more bonds to form. To minimize formal charge oxygen wants 2 bonds. Identify the atom that wants additional bonds and the number of electrons remaining on each atom: Atoms with large electronegativities can minimize their formal charge by forcing atoms with smaller electronegativities on period 3 or higher to expand their valence shells. The electronegativities of the atoms are 2.20 (hydrogen), 2.66 (iodine), and 3.44 (oxygen). Because the electronegativity of iodine is smaller than the electronegativity of oxygen, expand the valence shell of iodine to 7 bonds: Answer: | |
Basic properties
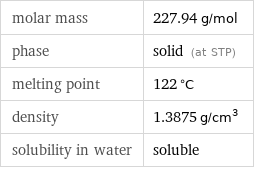
molar mass | 227.94 g/mol phase | solid (at STP) melting point | 122 °C density | 1.3875 g/cm^3 solubility in water | soluble
Units

Solid properties (at STP)
density | 1.3875 g/cm^3
Units

Chemical identifiers

CAS number | 10450-60-9 PubChem CID number | 25289 PubChem SID number | 24863698 SMILES identifier | OI(=O)(O)(O)(O)O InChI identifier | InChI=1/H5IO6/c2-1(3, 4, 5, 6)7/h(H5, 2, 3, 4, 5, 6, 7)/f/h2-6H MDL number | MFCD00011344
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 1
Toxicity properties

odor | odorless