Input interpretation
group 17 ions
Members
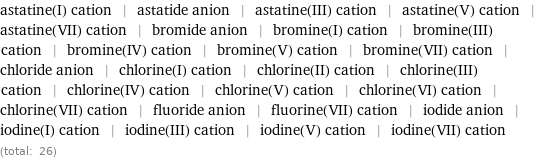
astatine(I) cation | astatide anion | astatine(III) cation | astatine(V) cation | astatine(VII) cation | bromide anion | bromine(I) cation | bromine(III) cation | bromine(IV) cation | bromine(V) cation | bromine(VII) cation | chloride anion | chlorine(I) cation | chlorine(II) cation | chlorine(III) cation | chlorine(IV) cation | chlorine(V) cation | chlorine(VI) cation | chlorine(VII) cation | fluoride anion | fluorine(VII) cation | iodide anion | iodine(I) cation | iodine(III) cation | iodine(V) cation | iodine(VII) cation (total: 26)
Ionic radii
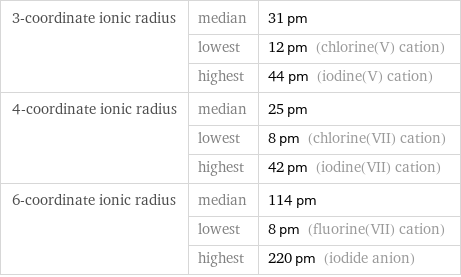
3-coordinate ionic radius | median | 31 pm | lowest | 12 pm (chlorine(V) cation) | highest | 44 pm (iodine(V) cation) 4-coordinate ionic radius | median | 25 pm | lowest | 8 pm (chlorine(VII) cation) | highest | 42 pm (iodine(VII) cation) 6-coordinate ionic radius | median | 114 pm | lowest | 8 pm (fluorine(VII) cation) | highest | 220 pm (iodide anion)
Units
