Input interpretation
magnesium fluoride | bis(trifluoromethylthio)mercury
Chemical names and formulas
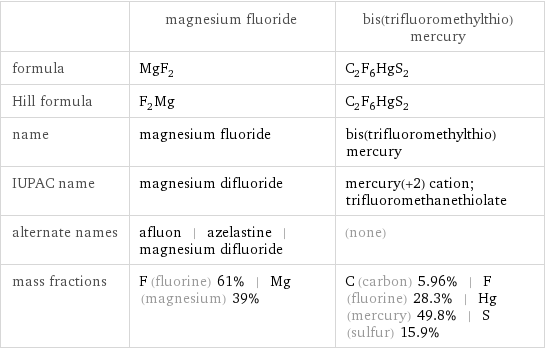
| magnesium fluoride | bis(trifluoromethylthio)mercury formula | MgF_2 | C_2F_6HgS_2 Hill formula | F_2Mg | C_2F_6HgS_2 name | magnesium fluoride | bis(trifluoromethylthio)mercury IUPAC name | magnesium difluoride | mercury(+2) cation; trifluoromethanethiolate alternate names | afluon | azelastine | magnesium difluoride | (none) mass fractions | F (fluorine) 61% | Mg (magnesium) 39% | C (carbon) 5.96% | F (fluorine) 28.3% | Hg (mercury) 49.8% | S (sulfur) 15.9%
Structure diagrams
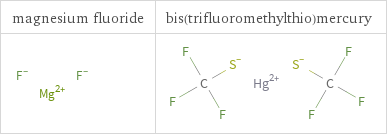
Structure diagrams
Basic properties
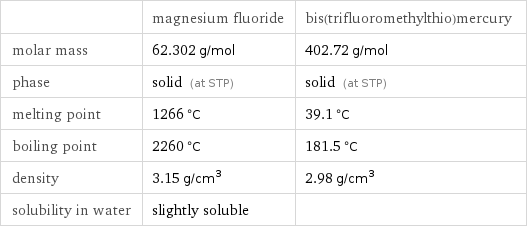
| magnesium fluoride | bis(trifluoromethylthio)mercury molar mass | 62.302 g/mol | 402.72 g/mol phase | solid (at STP) | solid (at STP) melting point | 1266 °C | 39.1 °C boiling point | 2260 °C | 181.5 °C density | 3.15 g/cm^3 | 2.98 g/cm^3 solubility in water | slightly soluble |
Units

Hydrophobicity and permeability properties
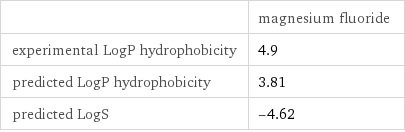
| magnesium fluoride experimental LogP hydrophobicity | 4.9 predicted LogP hydrophobicity | 3.81 predicted LogS | -4.62
Thermodynamic properties
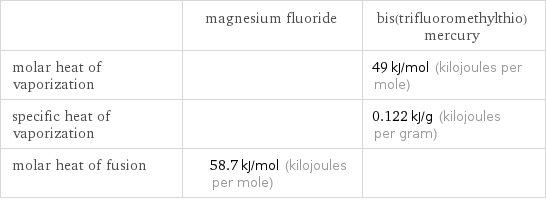
| magnesium fluoride | bis(trifluoromethylthio)mercury molar heat of vaporization | | 49 kJ/mol (kilojoules per mole) specific heat of vaporization | | 0.122 kJ/g (kilojoules per gram) molar heat of fusion | 58.7 kJ/mol (kilojoules per mole) |
Solid properties (at STP)

| magnesium fluoride | bis(trifluoromethylthio)mercury density | 3.15 g/cm^3 | 2.98 g/cm^3 vapor pressure | | 2.4 mmHg
Units

Chemical identifiers
![| magnesium fluoride | bis(trifluoromethylthio)mercury CAS number | 7783-40-6 | 21259-75-6 Beilstein number | | 3688992 PubChem CID number | 24546 | 30554 PubChem SID number | 9970 | SMILES identifier | [F-].[F-].[Mg+2] | C(F)(F)(F)[S-].C(F)(F)(F)[S-].[Hg+2]](../image_source/7490821239aa1ef600e4fa61ed54d300.png)
| magnesium fluoride | bis(trifluoromethylthio)mercury CAS number | 7783-40-6 | 21259-75-6 Beilstein number | | 3688992 PubChem CID number | 24546 | 30554 PubChem SID number | 9970 | SMILES identifier | [F-].[F-].[Mg+2] | C(F)(F)(F)[S-].C(F)(F)(F)[S-].[Hg+2]