Input interpretation

sodium bromate | tellurium hexafluoride
Chemical names and formulas
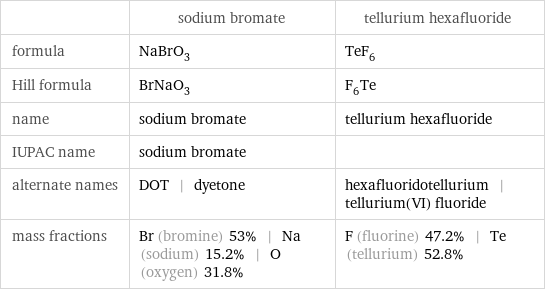
| sodium bromate | tellurium hexafluoride formula | NaBrO_3 | TeF_6 Hill formula | BrNaO_3 | F_6Te name | sodium bromate | tellurium hexafluoride IUPAC name | sodium bromate | alternate names | DOT | dyetone | hexafluoridotellurium | tellurium(VI) fluoride mass fractions | Br (bromine) 53% | Na (sodium) 15.2% | O (oxygen) 31.8% | F (fluorine) 47.2% | Te (tellurium) 52.8%
Structure diagrams
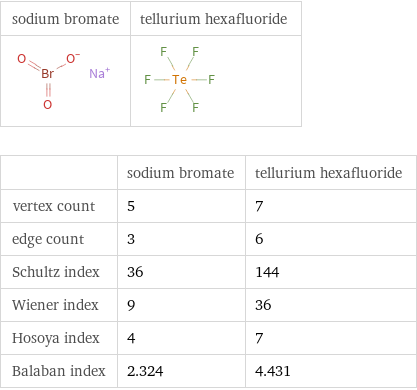
| sodium bromate | tellurium hexafluoride vertex count | 5 | 7 edge count | 3 | 6 Schultz index | 36 | 144 Wiener index | 9 | 36 Hosoya index | 4 | 7 Balaban index | 2.324 | 4.431
Basic properties
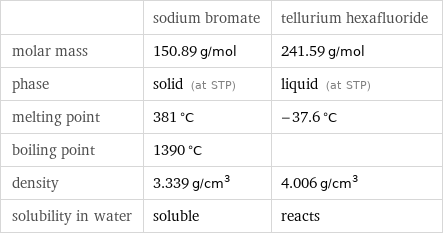
| sodium bromate | tellurium hexafluoride molar mass | 150.89 g/mol | 241.59 g/mol phase | solid (at STP) | liquid (at STP) melting point | 381 °C | -37.6 °C boiling point | 1390 °C | density | 3.339 g/cm^3 | 4.006 g/cm^3 solubility in water | soluble | reacts
Units

Liquid properties
| tellurium hexafluoride density | 4.006 g/cm^3
Units

Thermodynamic properties
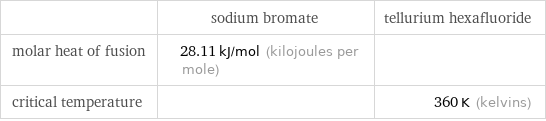
| sodium bromate | tellurium hexafluoride molar heat of fusion | 28.11 kJ/mol (kilojoules per mole) | critical temperature | | 360 K (kelvins)
Solid properties
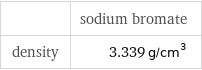
| sodium bromate density | 3.339 g/cm^3
Units

Chemical identifiers
![| sodium bromate | tellurium hexafluoride CAS number | 7789-38-0 | 7783-80-4 PubChem CID number | 23668195 | 24559 PubChem SID number | 24853461 | SMILES identifier | [O-]Br(=O)=O.[Na+] | F[Te](F)(F)(F)(F)F](../image_source/6b6e5038550c937a06a8ab1afe8b5247.png)
| sodium bromate | tellurium hexafluoride CAS number | 7789-38-0 | 7783-80-4 PubChem CID number | 23668195 | 24559 PubChem SID number | 24853461 | SMILES identifier | [O-]Br(=O)=O.[Na+] | F[Te](F)(F)(F)(F)F