Input interpretation
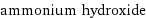
ammonium hydroxide
Chemical names and formulas

formula | NH_4OH Hill formula | H_5NO name | ammonium hydroxide alternate names | ammonia, aqua | ammonia, aqueous | ammonia aqueous | ammonia solution | ammonia water | aqua ammonia | aqueous ammonia | azanium hydroxide mass fractions | H (hydrogen) 14.4% | N (nitrogen) 40% | O (oxygen) 45.7%
Structure diagram
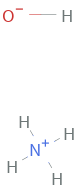
Structure diagram
Basic properties
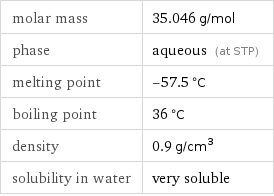
molar mass | 35.046 g/mol phase | aqueous (at STP) melting point | -57.5 °C boiling point | 36 °C density | 0.9 g/cm^3 solubility in water | very soluble
Units

Thermodynamic properties
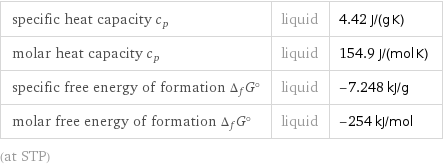
specific heat capacity c_p | liquid | 4.42 J/(g K) molar heat capacity c_p | liquid | 154.9 J/(mol K) specific free energy of formation Δ_fG° | liquid | -7.248 kJ/g molar free energy of formation Δ_fG° | liquid | -254 kJ/mol (at STP)
Chemical identifiers
![CAS number | 1336-21-6 Beilstein number | 3587154 PubChem CID number | 14923 PubChem SID number | 24859136 SMILES identifier | [NH4+].[OH-] InChI identifier | InChI=1/H3N.H2O/h1H3;1H2/fH4N.HO/h1H;1h/q+1;-1 MDL number | MFCD00066650](../image_source/6ff6703c63596a32ac2399775e1a37ce.png)
CAS number | 1336-21-6 Beilstein number | 3587154 PubChem CID number | 14923 PubChem SID number | 24859136 SMILES identifier | [NH4+].[OH-] InChI identifier | InChI=1/H3N.H2O/h1H3;1H2/fH4N.HO/h1H;1h/q+1;-1 MDL number | MFCD00066650
NFPA label

NFPA label

NFPA health rating | 3 NFPA fire rating | 0 NFPA reactivity rating | 0
Safety properties
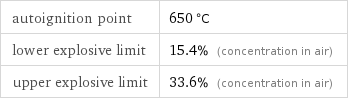
autoignition point | 650 °C lower explosive limit | 15.4% (concentration in air) upper explosive limit | 33.6% (concentration in air)
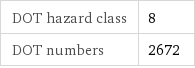
DOT hazard class | 8 DOT numbers | 2672
Toxicity properties

RTECS classes | mutagen | human data | primary irritant
Ion equivalents

(NH_4)^+ (ammonium cation) | 1 (OH)^- (hydroxide anion) | 1