Input interpretation

insulators | atomic radius
Summary
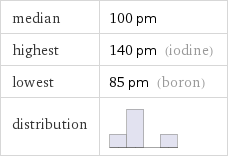
median | 100 pm highest | 140 pm (iodine) lowest | 85 pm (boron) distribution |
Units

Ranked values
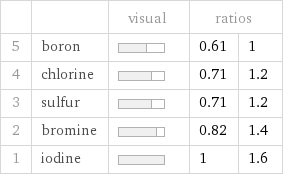
| | visual | ratios | 5 | boron | | 0.61 | 1 4 | chlorine | | 0.71 | 1.2 3 | sulfur | | 0.71 | 1.2 2 | bromine | | 0.82 | 1.4 1 | iodine | | 1 | 1.6
Plot
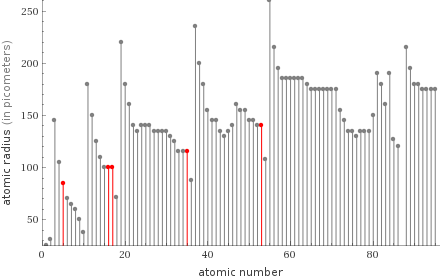
Plot
Periodic table location
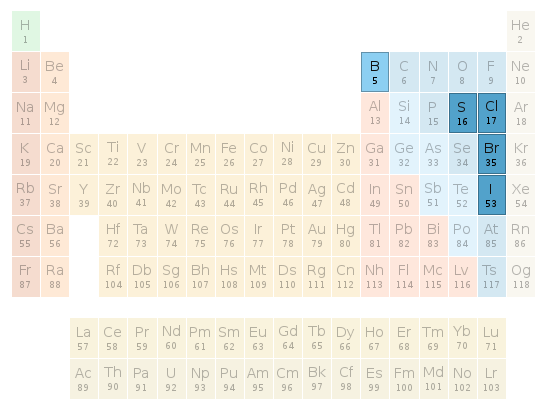
Periodic table location
Atomic radii properties
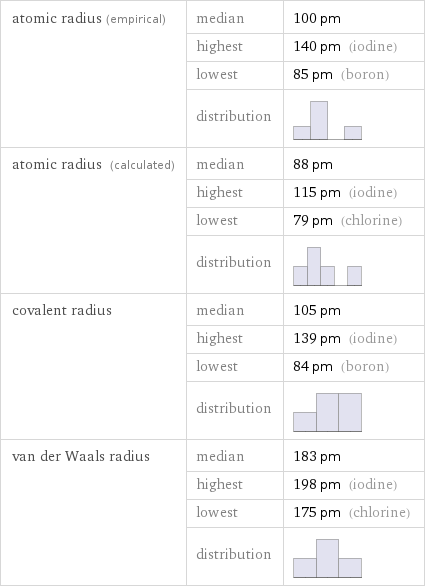
atomic radius (empirical) | median | 100 pm | highest | 140 pm (iodine) | lowest | 85 pm (boron) | distribution | atomic radius (calculated) | median | 88 pm | highest | 115 pm (iodine) | lowest | 79 pm (chlorine) | distribution | covalent radius | median | 105 pm | highest | 139 pm (iodine) | lowest | 84 pm (boron) | distribution | van der Waals radius | median | 183 pm | highest | 198 pm (iodine) | lowest | 175 pm (chlorine) | distribution |
Units

Atomic radius rankings
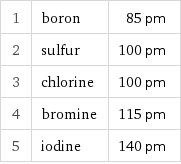
1 | boron | 85 pm 2 | sulfur | 100 pm 3 | chlorine | 100 pm 4 | bromine | 115 pm 5 | iodine | 140 pm
Unit conversions for median atomic radius 100 pm

0.1 nm (nanometers)
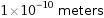
1×10^-10 meters
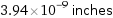
3.94×10^-9 inches
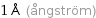
1 Å (ångström)
Comparisons for median atomic radius 100 pm
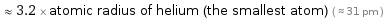
≈ 3.2 × atomic radius of helium (the smallest atom) ( ≈ 31 pm )
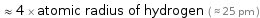
≈ 4 × atomic radius of hydrogen ( ≈ 25 pm )