Input interpretation

carbon allotropes | molar mass
Summary
![median | 12.011 g/mol highest | 840.77 g/mol ([5, 6]-fullerene-C70) lowest | 12.011 g/mol (3 chemicals) distribution |](../image_source/200d9dbb319cd6dada10fcab4ab86c38.png)
median | 12.011 g/mol highest | 840.77 g/mol ([5, 6]-fullerene-C70) lowest | 12.011 g/mol (3 chemicals) distribution |
Units

Ranked values
![| | visual | ratios | 5 | activated charcoal | | 0.014286 | 1 4 | diamond | | 0.014286 | 1 3 | graphite | | 0.014286 | 1 2 | buckminsterfullerene | | 0.8571 | 60 1 | [5, 6]-fullerene-C70 | | 1 | 70](../image_source/25896aa60129effa3917a57f45e52d25.png)
| | visual | ratios | 5 | activated charcoal | | 0.014286 | 1 4 | diamond | | 0.014286 | 1 3 | graphite | | 0.014286 | 1 2 | buckminsterfullerene | | 0.8571 | 60 1 | [5, 6]-fullerene-C70 | | 1 | 70
Molar mass rankings
![1 | graphite | 12.011 g/mol 2 | diamond | 12.011 g/mol 3 | activated charcoal | 12.011 g/mol 4 | buckminsterfullerene | 720.66 g/mol 5 | [5, 6]-fullerene-C70 | 840.77 g/mol](../image_source/15b3271460db60e067b5e5dc1528ab59.png)
1 | graphite | 12.011 g/mol 2 | diamond | 12.011 g/mol 3 | activated charcoal | 12.011 g/mol 4 | buckminsterfullerene | 720.66 g/mol 5 | [5, 6]-fullerene-C70 | 840.77 g/mol
Unit conversion for median molar mass 12.011 g/mol
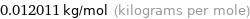
0.012011 kg/mol (kilograms per mole)
Comparisons for median molar mass 12.011 g/mol
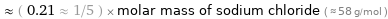
≈ ( 0.21 ≈ 1/5 ) × molar mass of sodium chloride ( ≈ 58 g/mol )
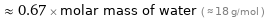
≈ 0.67 × molar mass of water ( ≈ 18 g/mol )
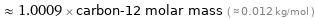
≈ 1.0009 × carbon-12 molar mass ( ≈ 0.012 kg/mol )
Corresponding quantities
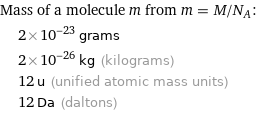
Mass of a molecule m from m = M/N_A: | 2×10^-23 grams | 2×10^-26 kg (kilograms) | 12 u (unified atomic mass units) | 12 Da (daltons)
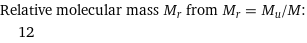
Relative molecular mass M_r from M_r = M_u/M: | 12