Input interpretation
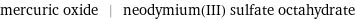
mercuric oxide | neodymium(III) sulfate octahydrate
Chemical names and formulas
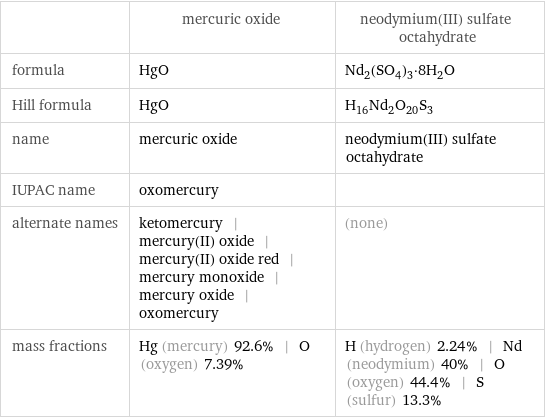
| mercuric oxide | neodymium(III) sulfate octahydrate formula | HgO | Nd_2(SO_4)_3·8H_2O Hill formula | HgO | H_16Nd_2O_20S_3 name | mercuric oxide | neodymium(III) sulfate octahydrate IUPAC name | oxomercury | alternate names | ketomercury | mercury(II) oxide | mercury(II) oxide red | mercury monoxide | mercury oxide | oxomercury | (none) mass fractions | Hg (mercury) 92.6% | O (oxygen) 7.39% | H (hydrogen) 2.24% | Nd (neodymium) 40% | O (oxygen) 44.4% | S (sulfur) 13.3%
Structure diagrams
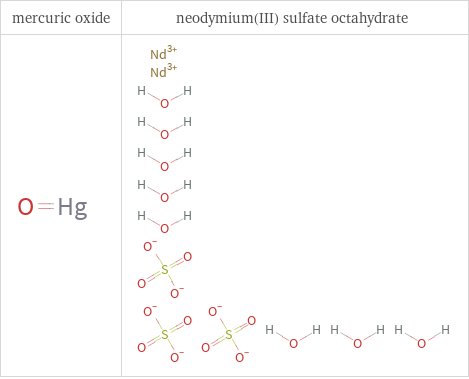
Structure diagrams
Basic properties
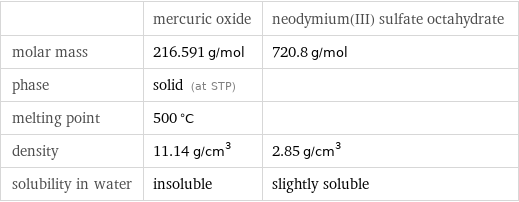
| mercuric oxide | neodymium(III) sulfate octahydrate molar mass | 216.591 g/mol | 720.8 g/mol phase | solid (at STP) | melting point | 500 °C | density | 11.14 g/cm^3 | 2.85 g/cm^3 solubility in water | insoluble | slightly soluble
Units

Solid properties

| mercuric oxide density | 11.14 g/cm^3 vapor pressure | 702.025 mmHg
Units
