Input interpretation
lanthanides | molar heat of vaporization
Summary
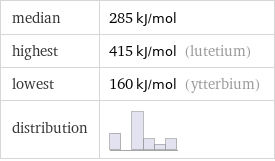
median | 285 kJ/mol highest | 415 kJ/mol (lutetium) lowest | 160 kJ/mol (ytterbium) distribution |
Units

Distribution plots
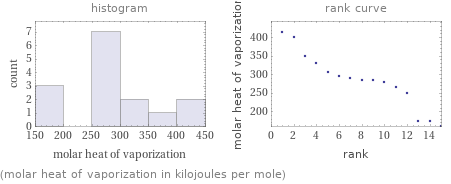
(molar heat of vaporization in kilojoules per mole)
Molar heat of vaporization rankings
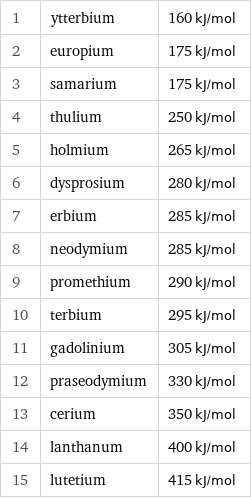
1 | ytterbium | 160 kJ/mol 2 | europium | 175 kJ/mol 3 | samarium | 175 kJ/mol 4 | thulium | 250 kJ/mol 5 | holmium | 265 kJ/mol 6 | dysprosium | 280 kJ/mol 7 | erbium | 285 kJ/mol 8 | neodymium | 285 kJ/mol 9 | promethium | 290 kJ/mol 10 | terbium | 295 kJ/mol 11 | gadolinium | 305 kJ/mol 12 | praseodymium | 330 kJ/mol 13 | cerium | 350 kJ/mol 14 | lanthanum | 400 kJ/mol 15 | lutetium | 415 kJ/mol
Unit conversions for median molar heat of vaporization 285 kJ/mol
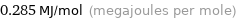
0.285 MJ/mol (megajoules per mole)
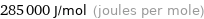
285000 J/mol (joules per mole)

68.1 kcal/mol (thermochemical kilocalories per mole)
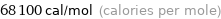
68100 cal/mol (calories per mole)
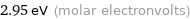
2.95 eV (molar electronvolts)