Input interpretation
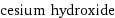
cesium hydroxide
Chemical names and formulas
formula | CsOH Hill formula | CsHO name | cesium hydroxide IUPAC name | cesium;hydroxide alternate names | caesium hydroxide | cesium hydrate | cesium hydroxide hydrate | cesium hydroxide, solution mass fractions | Cs (cesium) 88.7% | H (hydrogen) 0.672% | O (oxygen) 10.7%
Structure diagram

Structure diagram
vertex count | 2 edge count | 1 Schultz index | 0 Wiener index | 0 Hosoya index | 1 Balaban index | 0
Basic properties
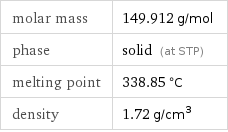
molar mass | 149.912 g/mol phase | solid (at STP) melting point | 338.85 °C density | 1.72 g/cm^3
Units

Solid properties (at STP)

density | 1.72 g/cm^3 vapor pressure | 934.43 mmHg (at 546.3 °C)
Units

Thermodynamic properties
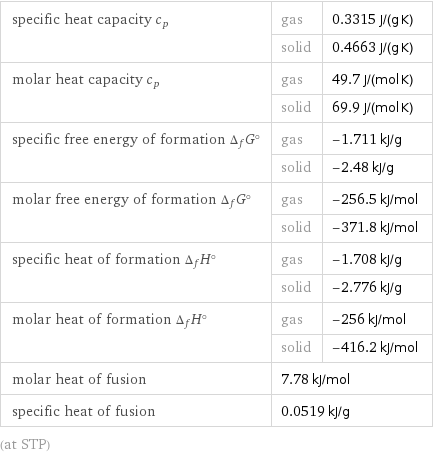
specific heat capacity c_p | gas | 0.3315 J/(g K) | solid | 0.4663 J/(g K) molar heat capacity c_p | gas | 49.7 J/(mol K) | solid | 69.9 J/(mol K) specific free energy of formation Δ_fG° | gas | -1.711 kJ/g | solid | -2.48 kJ/g molar free energy of formation Δ_fG° | gas | -256.5 kJ/mol | solid | -371.8 kJ/mol specific heat of formation Δ_fH° | gas | -1.708 kJ/g | solid | -2.776 kJ/g molar heat of formation Δ_fH° | gas | -256 kJ/mol | solid | -416.2 kJ/mol molar heat of fusion | 7.78 kJ/mol | specific heat of fusion | 0.0519 kJ/g | (at STP)
Chemical identifiers
![CAS number | 21351-79-1 PubChem CID number | 62750 SMILES identifier | [OH-].[Cs+] MDL number | MFCD00010964](../image_source/c3dbcce0b1aea601c5dedb5bd7e98b86.png)
CAS number | 21351-79-1 PubChem CID number | 62750 SMILES identifier | [OH-].[Cs+] MDL number | MFCD00010964
Toxicity properties

RTECS classes | primary irritant