Input interpretation

metallic elements | van der Waals radius
Summary
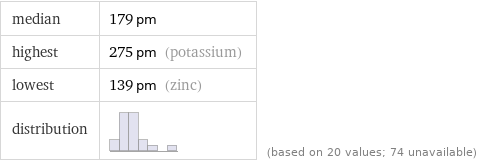
median | 179 pm highest | 275 pm (potassium) lowest | 139 pm (zinc) distribution | | (based on 20 values; 74 unavailable)
Entities with missing values

beryllium | aluminum | calcium | scandium | titanium | vanadium | chromium | manganese | iron | cobalt | ... (total: 74)
Plot
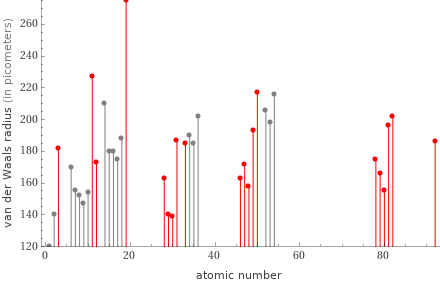
Plot
Periodic table location
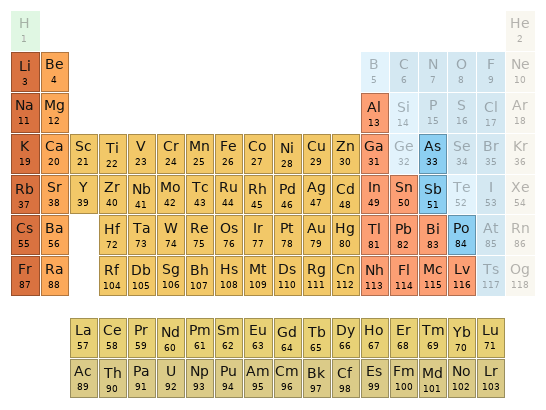
Periodic table location
Atomic radii properties
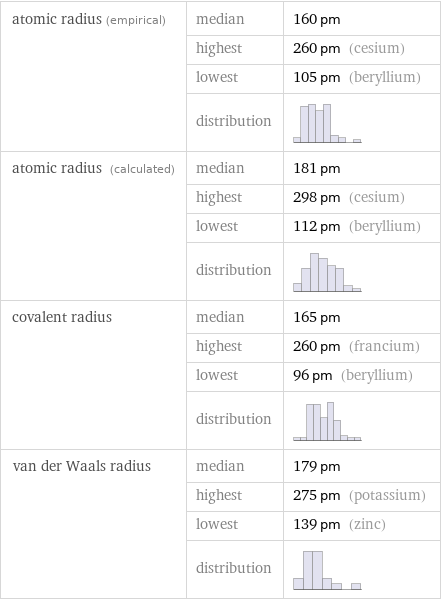
atomic radius (empirical) | median | 160 pm | highest | 260 pm (cesium) | lowest | 105 pm (beryllium) | distribution | atomic radius (calculated) | median | 181 pm | highest | 298 pm (cesium) | lowest | 112 pm (beryllium) | distribution | covalent radius | median | 165 pm | highest | 260 pm (francium) | lowest | 96 pm (beryllium) | distribution | van der Waals radius | median | 179 pm | highest | 275 pm (potassium) | lowest | 139 pm (zinc) | distribution |
Units

Distribution plots
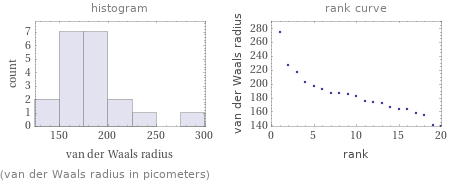
(van der Waals radius in picometers)
Van der Waals radius rankings
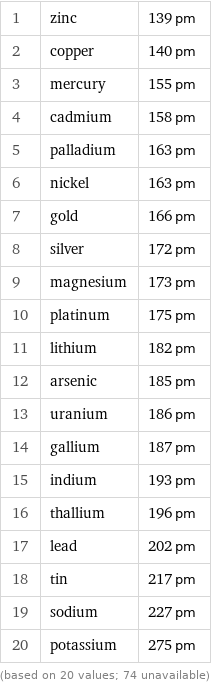
1 | zinc | 139 pm 2 | copper | 140 pm 3 | mercury | 155 pm 4 | cadmium | 158 pm 5 | palladium | 163 pm 6 | nickel | 163 pm 7 | gold | 166 pm 8 | silver | 172 pm 9 | magnesium | 173 pm 10 | platinum | 175 pm 11 | lithium | 182 pm 12 | arsenic | 185 pm 13 | uranium | 186 pm 14 | gallium | 187 pm 15 | indium | 193 pm 16 | thallium | 196 pm 17 | lead | 202 pm 18 | tin | 217 pm 19 | sodium | 227 pm 20 | potassium | 275 pm (based on 20 values; 74 unavailable)
Unit conversions for median van der Waals radius 179 pm
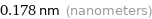
0.178 nm (nanometers)
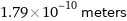
1.79×10^-10 meters
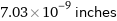
7.03×10^-9 inches
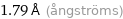
1.79 Å (ångströms)
Comparisons for median van der Waals radius 179 pm
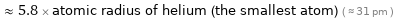
≈ 5.8 × atomic radius of helium (the smallest atom) ( ≈ 31 pm )
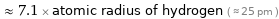
≈ 7.1 × atomic radius of hydrogen ( ≈ 25 pm )