Input interpretation
group ions
Members

cesium cation | lithium cation | potassium cation | rubidium cation | sodium cation | barium cation | beryllium cation | calcium cation | magnesium cation | radium cation | strontium cation | scandium(III) cation | yttrium(III) cation | titanium(II) cation | titanium(III) cation | titanium(IV) cation | zirconium(IV) cation | niobium(III) cation | vanadium(II) cation | vanadium(III) cation | chromium(II) cation | chromium(III) cation | chromium(VI) cation | molybdenum(III) cation | manganese(V) cation | manganese(II) cation | manganese(III) cation | manganese(IV) cation | manganese(VII) cation | iron(II) cation | iron(III) cation | ruthenium(I) cation | ruthenium(II) cation | ruthenium(III) cation | cobalt(II) cation | cobalt(III) cation | nickel(II) cation | nickel(III) cation | palladium(I) cation | palladium(II) cation | ... (total: 81)
Ionic radii
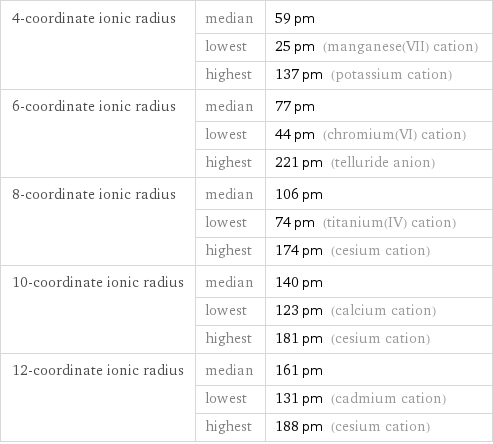
4-coordinate ionic radius | median | 59 pm | lowest | 25 pm (manganese(VII) cation) | highest | 137 pm (potassium cation) 6-coordinate ionic radius | median | 77 pm | lowest | 44 pm (chromium(VI) cation) | highest | 221 pm (telluride anion) 8-coordinate ionic radius | median | 106 pm | lowest | 74 pm (titanium(IV) cation) | highest | 174 pm (cesium cation) 10-coordinate ionic radius | median | 140 pm | lowest | 123 pm (calcium cation) | highest | 181 pm (cesium cation) 12-coordinate ionic radius | median | 161 pm | lowest | 131 pm (cadmium cation) | highest | 188 pm (cesium cation)
Units
