Input interpretation

elements | with density<12 g/cm^3 (grams per cubic centimeter)
Result

thallium | thorium | technetium | lead | silver | molybdenum | actinium | lutetium | bismuth | thulium | polonium | erbium | copper | nickel | cobalt | holmium | cadmium | niobium | dysprosium | terbium | gadolinium | iron | manganese | samarium | indium | tin | promethium | chromium | zinc | neodymium | antimony | cerium | praseodymium | ytterbium | zirconium | tellurium | lanthanum | vanadium | gallium | arsenic | ... (total: 76)
Periodic table location
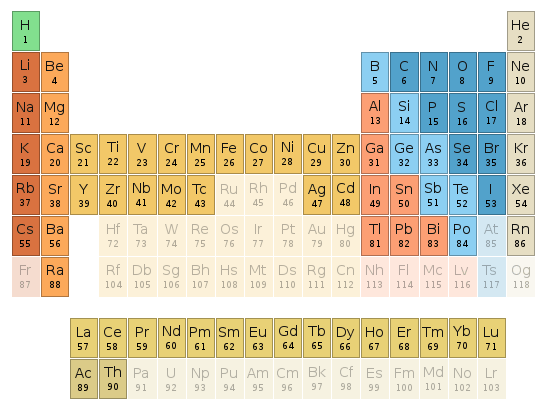
Periodic table location
Images
Images
Basic elemental properties
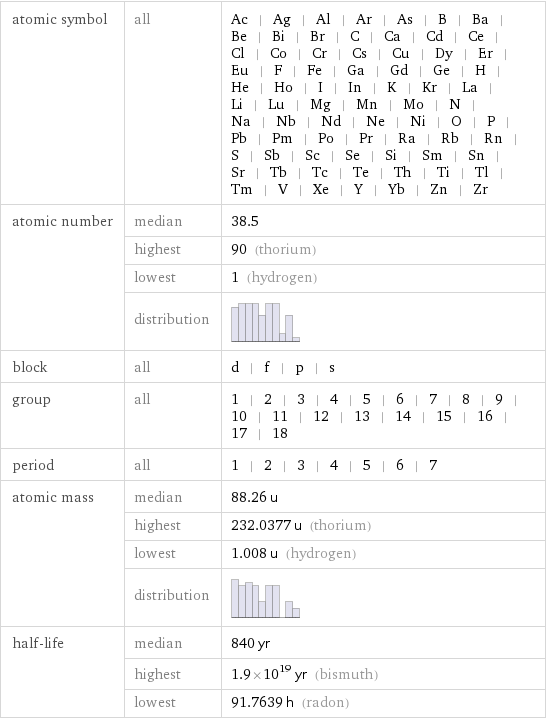
atomic symbol | all | Ac | Ag | Al | Ar | As | B | Ba | Be | Bi | Br | C | Ca | Cd | Ce | Cl | Co | Cr | Cs | Cu | Dy | Er | Eu | F | Fe | Ga | Gd | Ge | H | He | Ho | I | In | K | Kr | La | Li | Lu | Mg | Mn | Mo | N | Na | Nb | Nd | Ne | Ni | O | P | Pb | Pm | Po | Pr | Ra | Rb | Rn | S | Sb | Sc | Se | Si | Sm | Sn | Sr | Tb | Tc | Te | Th | Ti | Tl | Tm | V | Xe | Y | Yb | Zn | Zr atomic number | median | 38.5 | highest | 90 (thorium) | lowest | 1 (hydrogen) | distribution | block | all | d | f | p | s group | all | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 period | all | 1 | 2 | 3 | 4 | 5 | 6 | 7 atomic mass | median | 88.26 u | highest | 232.0377 u (thorium) | lowest | 1.008 u (hydrogen) | distribution | half-life | median | 840 yr | highest | 1.9×10^19 yr (bismuth) | lowest | 91.7639 h (radon)
Thermodynamic properties
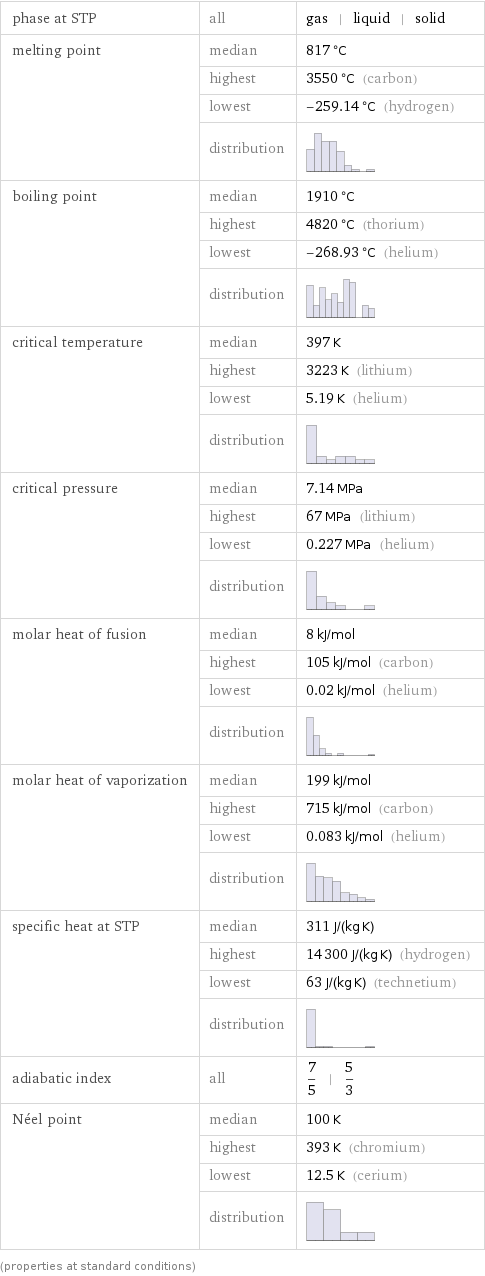
phase at STP | all | gas | liquid | solid melting point | median | 817 °C | highest | 3550 °C (carbon) | lowest | -259.14 °C (hydrogen) | distribution | boiling point | median | 1910 °C | highest | 4820 °C (thorium) | lowest | -268.93 °C (helium) | distribution | critical temperature | median | 397 K | highest | 3223 K (lithium) | lowest | 5.19 K (helium) | distribution | critical pressure | median | 7.14 MPa | highest | 67 MPa (lithium) | lowest | 0.227 MPa (helium) | distribution | molar heat of fusion | median | 8 kJ/mol | highest | 105 kJ/mol (carbon) | lowest | 0.02 kJ/mol (helium) | distribution | molar heat of vaporization | median | 199 kJ/mol | highest | 715 kJ/mol (carbon) | lowest | 0.083 kJ/mol (helium) | distribution | specific heat at STP | median | 311 J/(kg K) | highest | 14300 J/(kg K) (hydrogen) | lowest | 63 J/(kg K) (technetium) | distribution | adiabatic index | all | 7/5 | 5/3 Néel point | median | 100 K | highest | 393 K (chromium) | lowest | 12.5 K (cerium) | distribution | (properties at standard conditions)