Input interpretation

period 4 elements | phase at STP
Summary
gas | liquid | solid
Table
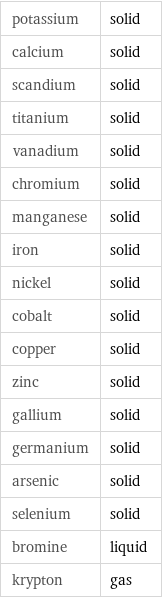
potassium | solid calcium | solid scandium | solid titanium | solid vanadium | solid chromium | solid manganese | solid iron | solid nickel | solid cobalt | solid copper | solid zinc | solid gallium | solid germanium | solid arsenic | solid selenium | solid bromine | liquid krypton | gas
Thermodynamic properties
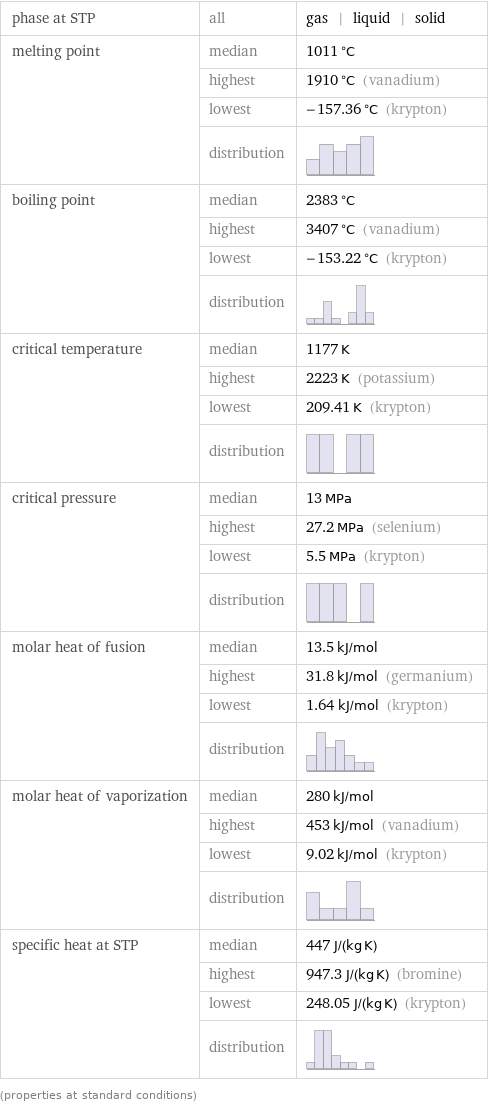
phase at STP | all | gas | liquid | solid melting point | median | 1011 °C | highest | 1910 °C (vanadium) | lowest | -157.36 °C (krypton) | distribution | boiling point | median | 2383 °C | highest | 3407 °C (vanadium) | lowest | -153.22 °C (krypton) | distribution | critical temperature | median | 1177 K | highest | 2223 K (potassium) | lowest | 209.41 K (krypton) | distribution | critical pressure | median | 13 MPa | highest | 27.2 MPa (selenium) | lowest | 5.5 MPa (krypton) | distribution | molar heat of fusion | median | 13.5 kJ/mol | highest | 31.8 kJ/mol (germanium) | lowest | 1.64 kJ/mol (krypton) | distribution | molar heat of vaporization | median | 280 kJ/mol | highest | 453 kJ/mol (vanadium) | lowest | 9.02 kJ/mol (krypton) | distribution | specific heat at STP | median | 447 J/(kg K) | highest | 947.3 J/(kg K) (bromine) | lowest | 248.05 J/(kg K) (krypton) | distribution | (properties at standard conditions)