Input interpretation

Cd(NO3)2 ⟶ HNO_3 nitric acid + CdOHNO3
Chemical names and formulas
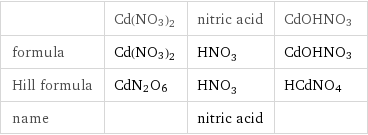
| Cd(NO3)2 | nitric acid | CdOHNO3 formula | Cd(NO3)2 | HNO_3 | CdOHNO3 Hill formula | CdN2O6 | HNO_3 | HCdNO4 name | | nitric acid |
Substance properties
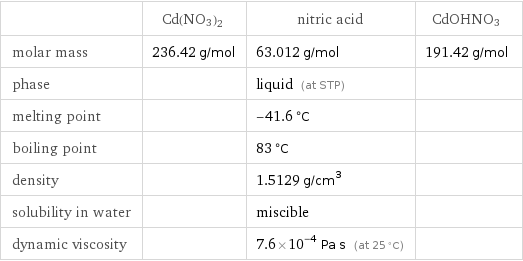
| Cd(NO3)2 | nitric acid | CdOHNO3 molar mass | 236.42 g/mol | 63.012 g/mol | 191.42 g/mol phase | | liquid (at STP) | melting point | | -41.6 °C | boiling point | | 83 °C | density | | 1.5129 g/cm^3 | solubility in water | | miscible | dynamic viscosity | | 7.6×10^-4 Pa s (at 25 °C) |
Units
