Input interpretation
zinc chloride
Chemical names and formulas
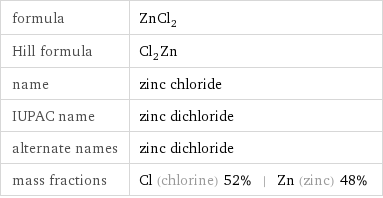
formula | ZnCl_2 Hill formula | Cl_2Zn name | zinc chloride IUPAC name | zinc dichloride alternate names | zinc dichloride mass fractions | Cl (chlorine) 52% | Zn (zinc) 48%
Structure diagram
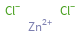
Structure diagram
Basic properties
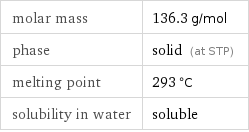
molar mass | 136.3 g/mol phase | solid (at STP) melting point | 293 °C solubility in water | soluble
Units

Input interpretation
zinc chloride
Solid properties (at STP)

vapor pressure | 0.9998 mmHg
Units

Chemical names and formulas
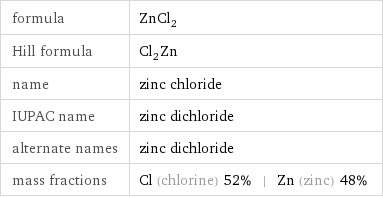
formula | ZnCl_2 Hill formula | Cl_2Zn name | zinc chloride IUPAC name | zinc dichloride alternate names | zinc dichloride mass fractions | Cl (chlorine) 52% | Zn (zinc) 48%
Structure diagram
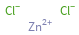
Structure diagram
Thermodynamic properties
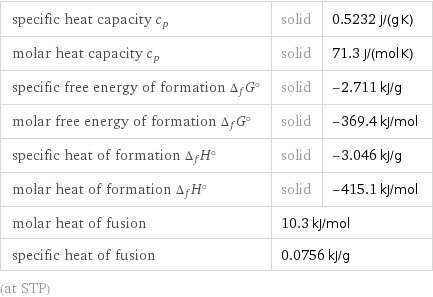
specific heat capacity c_p | solid | 0.5232 J/(g K) molar heat capacity c_p | solid | 71.3 J/(mol K) specific free energy of formation Δ_fG° | solid | -2.711 kJ/g molar free energy of formation Δ_fG° | solid | -369.4 kJ/mol specific heat of formation Δ_fH° | solid | -3.046 kJ/g molar heat of formation Δ_fH° | solid | -415.1 kJ/mol molar heat of fusion | 10.3 kJ/mol | specific heat of fusion | 0.0756 kJ/g | (at STP)
Basic properties
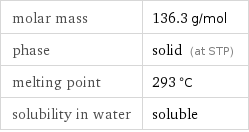
molar mass | 136.3 g/mol phase | solid (at STP) melting point | 293 °C solubility in water | soluble
Units

Chemical identifiers
![CAS number | 7646-85-7 PubChem CID number | 3007855 PubChem SID number | 24853766 SMILES identifier | [Cl-].[Cl-].[Zn+2] InChI identifier | InChI=1/2ClH.Zn/h2*1H;/q;;+2/p-2/f2Cl.Zn/h2*1h;/q2*-1;m RTECS number | ZH1400000 MDL number | MFCD00011295](../image_source/9e3146873dedc55b6b518cab3646631b.png)
CAS number | 7646-85-7 PubChem CID number | 3007855 PubChem SID number | 24853766 SMILES identifier | [Cl-].[Cl-].[Zn+2] InChI identifier | InChI=1/2ClH.Zn/h2*1H;/q;;+2/p-2/f2Cl.Zn/h2*1h;/q2*-1;m RTECS number | ZH1400000 MDL number | MFCD00011295
Toxicity properties

odor | odorless short-term exposure limit | 2 mg/m^3

long-term exposure limit | 1 mg/m^3 (over 8 hours)
Solid properties (at STP)

vapor pressure | 0.9998 mmHg
Units

Ion equivalents

Zn^(2+) (zinc cation) | 1 Cl^- (chloride anion) | 2
Thermodynamic properties
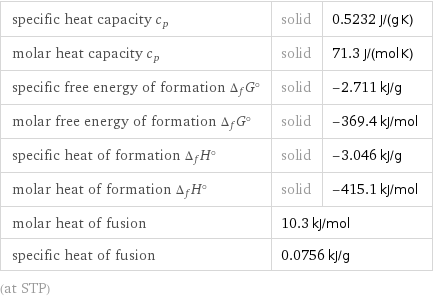
specific heat capacity c_p | solid | 0.5232 J/(g K) molar heat capacity c_p | solid | 71.3 J/(mol K) specific free energy of formation Δ_fG° | solid | -2.711 kJ/g molar free energy of formation Δ_fG° | solid | -369.4 kJ/mol specific heat of formation Δ_fH° | solid | -3.046 kJ/g molar heat of formation Δ_fH° | solid | -415.1 kJ/mol molar heat of fusion | 10.3 kJ/mol | specific heat of fusion | 0.0756 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7646-85-7 PubChem CID number | 3007855 PubChem SID number | 24853766 SMILES identifier | [Cl-].[Cl-].[Zn+2] InChI identifier | InChI=1/2ClH.Zn/h2*1H;/q;;+2/p-2/f2Cl.Zn/h2*1h;/q2*-1;m RTECS number | ZH1400000 MDL number | MFCD00011295](../image_source/4f9caadd4a7c80e366b361f001362caf.png)
CAS number | 7646-85-7 PubChem CID number | 3007855 PubChem SID number | 24853766 SMILES identifier | [Cl-].[Cl-].[Zn+2] InChI identifier | InChI=1/2ClH.Zn/h2*1H;/q;;+2/p-2/f2Cl.Zn/h2*1h;/q2*-1;m RTECS number | ZH1400000 MDL number | MFCD00011295
Toxicity properties

odor | odorless short-term exposure limit | 2 mg/m^3

long-term exposure limit | 1 mg/m^3 (over 8 hours)
Ion equivalents

Zn^(2+) (zinc cation) | 1 Cl^- (chloride anion) | 2
Input interpretation
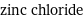
zinc chloride
Chemical names and formulas
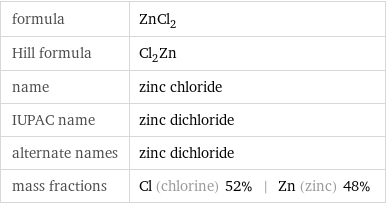
formula | ZnCl_2 Hill formula | Cl_2Zn name | zinc chloride IUPAC name | zinc dichloride alternate names | zinc dichloride mass fractions | Cl (chlorine) 52% | Zn (zinc) 48%
Structure diagram
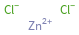
Structure diagram
Basic properties
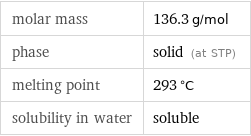
molar mass | 136.3 g/mol phase | solid (at STP) melting point | 293 °C solubility in water | soluble
Units

Solid properties (at STP)

vapor pressure | 0.9998 mmHg
Units

Thermodynamic properties
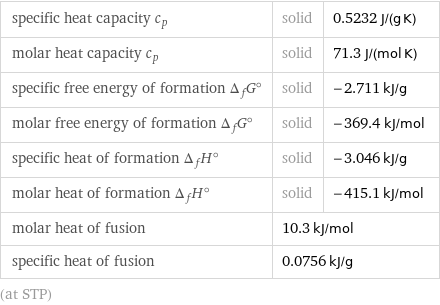
specific heat capacity c_p | solid | 0.5232 J/(g K) molar heat capacity c_p | solid | 71.3 J/(mol K) specific free energy of formation Δ_fG° | solid | -2.711 kJ/g molar free energy of formation Δ_fG° | solid | -369.4 kJ/mol specific heat of formation Δ_fH° | solid | -3.046 kJ/g molar heat of formation Δ_fH° | solid | -415.1 kJ/mol molar heat of fusion | 10.3 kJ/mol | specific heat of fusion | 0.0756 kJ/g | (at STP)
Chemical identifiers
![CAS number | 7646-85-7 PubChem CID number | 3007855 PubChem SID number | 24853766 SMILES identifier | [Cl-].[Cl-].[Zn+2] InChI identifier | InChI=1/2ClH.Zn/h2*1H;/q;;+2/p-2/f2Cl.Zn/h2*1h;/q2*-1;m RTECS number | ZH1400000 MDL number | MFCD00011295](../image_source/86abdf221f473b5db13d73546f652def.png)
CAS number | 7646-85-7 PubChem CID number | 3007855 PubChem SID number | 24853766 SMILES identifier | [Cl-].[Cl-].[Zn+2] InChI identifier | InChI=1/2ClH.Zn/h2*1H;/q;;+2/p-2/f2Cl.Zn/h2*1h;/q2*-1;m RTECS number | ZH1400000 MDL number | MFCD00011295
Toxicity properties

odor | odorless short-term exposure limit | 2 mg/m^3

long-term exposure limit | 1 mg/m^3 (over 8 hours)
Ion equivalents
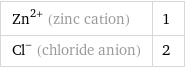
Zn^(2+) (zinc cation) | 1 Cl^- (chloride anion) | 2