Input interpretation
norethindrone
Chemical names and formulas
![formula | C_20H_26O_2 name | norethindrone IUPAC name | 17-ethynyl-17-hydroxy-13-methyl-1, 2, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16-dodecahydrocyclopenta[a]phenanthren-3-one alternate names | anovule | conludaf | ethinylnortestosterone | norethisterone mass fractions | C (carbon) 80.5% | H (hydrogen) 8.78% | O (oxygen) 10.7%](../image_source/5f4edb184f11861cf4860635a40d1e2d.png)
formula | C_20H_26O_2 name | norethindrone IUPAC name | 17-ethynyl-17-hydroxy-13-methyl-1, 2, 6, 7, 8, 9, 10, 11, 12, 14, 15, 16-dodecahydrocyclopenta[a]phenanthren-3-one alternate names | anovule | conludaf | ethinylnortestosterone | norethisterone mass fractions | C (carbon) 80.5% | H (hydrogen) 8.78% | O (oxygen) 10.7%
Lewis structure
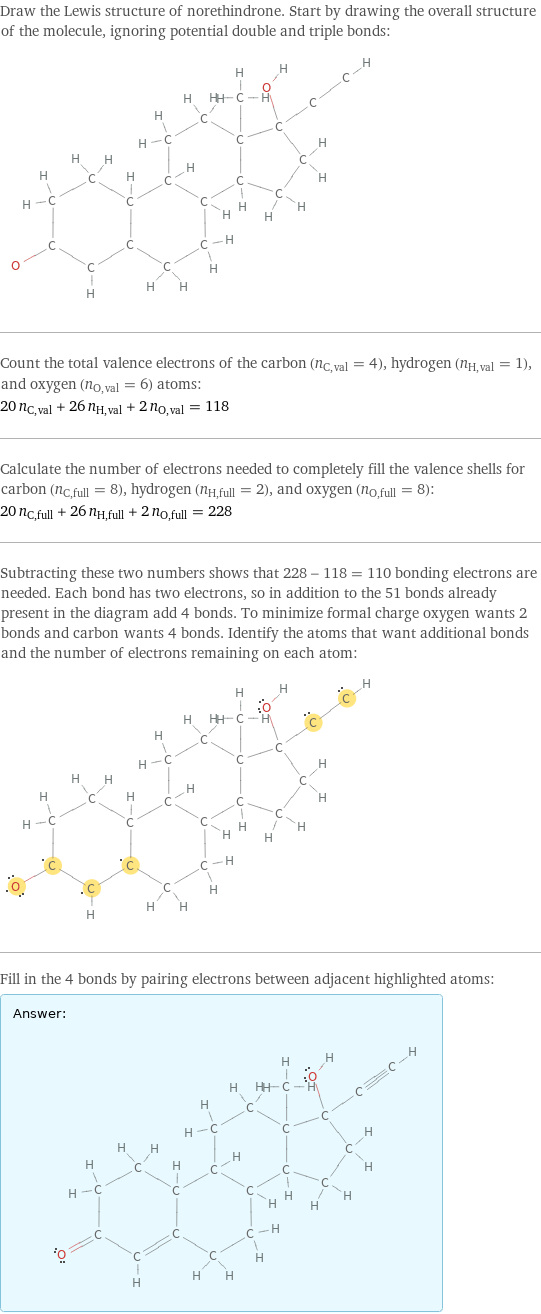
Draw the Lewis structure of norethindrone. Start by drawing the overall structure of the molecule, ignoring potential double and triple bonds: Count the total valence electrons of the carbon (n_C, val = 4), hydrogen (n_H, val = 1), and oxygen (n_O, val = 6) atoms: 20 n_C, val + 26 n_H, val + 2 n_O, val = 118 Calculate the number of electrons needed to completely fill the valence shells for carbon (n_C, full = 8), hydrogen (n_H, full = 2), and oxygen (n_O, full = 8): 20 n_C, full + 26 n_H, full + 2 n_O, full = 228 Subtracting these two numbers shows that 228 - 118 = 110 bonding electrons are needed. Each bond has two electrons, so in addition to the 51 bonds already present in the diagram add 4 bonds. To minimize formal charge oxygen wants 2 bonds and carbon wants 4 bonds. Identify the atoms that want additional bonds and the number of electrons remaining on each atom: Fill in the 4 bonds by pairing electrons between adjacent highlighted atoms: Answer: | |
3D structure
3D structure
Basic properties
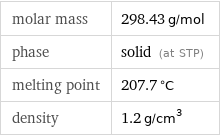
molar mass | 298.43 g/mol phase | solid (at STP) melting point | 207.7 °C density | 1.2 g/cm^3
Units

Hydrophobicity and permeability properties

experimental LogP hydrophobicity | 3.2 predicted LogP hydrophobicity | 2.72 experimental LogS | -4.57 predicted LogS | -4.65
Drug interactions

acitretin | amobarbital | allylpropymal | bosentan | butabarbital | butalbital | butethal | carbamazepine | ethotoin | fosphenytoin | griseofulvin | heptabarbital | hexobarbital | lamotrigine | mephenytoin | methohexital | methylphenobarbital | pentobarbital | phenobarbital | phenytoin | ... (total: 30)
Basic drug properties

approval status | approved | small molecule drug categories | synthetic oral contraceptive | progestin dosage forms | oral: tablet
![brand names | anhydrohydroxynorprogesterone | anovulatorio | anovule | aygestin | binovum | brevicon | brevinor | brevinor 21 | brevinor 28 | brevinor-1 21 | brevinor-1 28 | camila | ciclovulan | conceplan | conludaf | conludag | demulen | ENT | errin | estrinor | ethinylnortestosterone | ethynylmortestosterone | ethynylnortestosterone | gencept | genora | gestest | jenest | jenest-28 | loestrin | menzol | microneth | micronett | micronor | micronovum | milli | mini-Pe | mini-pill | minovlar | modicon | N.E.E. | NET | necon | nelova | neocon | nodiol | nor-Q.D. | nor-Qd | noraethisteronum | noralutin | norcept | norcept-E | norcolut | noresthisterone | norethadrone | norethin | norethin 1/35 E | norethin 1/50 M | norethindrone acetate | norethindrone norethisterone | norethisteron | norethisterone | norethisterone [progestins] | norethyndron | norethynodron | norethynodrone | noretisterone [dcit] | norfor | norgestin | noriday | noriday 28 | norimin | norinyl | norlestrin | norlutate | norluten | norlutin | norluton | normapause | norpregneninlone | norpregneninolone | norpregneninotone | orlest | ortho 1 35 | ortho 7 7 7 | ortho-novum | ortho-novum 1 35 | ortho-novum 1 50 | ortho-novum 7 7 7 | ovcon | ovysmen | ovysmen 0.5 35 | ovysmen 1 35 | palonyl | perovex | primolut N | primolut-N | proluteasi | synphase | synphasic 28 | tri-norinyl | triella | trinovum | trinovum 21 | utovlan | utovlar](../image_source/68df71a9f43db07e37e837daa40b0316.png)
brand names | anhydrohydroxynorprogesterone | anovulatorio | anovule | aygestin | binovum | brevicon | brevinor | brevinor 21 | brevinor 28 | brevinor-1 21 | brevinor-1 28 | camila | ciclovulan | conceplan | conludaf | conludag | demulen | ENT | errin | estrinor | ethinylnortestosterone | ethynylmortestosterone | ethynylnortestosterone | gencept | genora | gestest | jenest | jenest-28 | loestrin | menzol | microneth | micronett | micronor | micronovum | milli | mini-Pe | mini-pill | minovlar | modicon | N.E.E. | NET | necon | nelova | neocon | nodiol | nor-Q.D. | nor-Qd | noraethisteronum | noralutin | norcept | norcept-E | norcolut | noresthisterone | norethadrone | norethin | norethin 1/35 E | norethin 1/50 M | norethindrone acetate | norethindrone norethisterone | norethisteron | norethisterone | norethisterone [progestins] | norethyndron | norethynodron | norethynodrone | noretisterone [dcit] | norfor | norgestin | noriday | noriday 28 | norimin | norinyl | norlestrin | norlutate | norluten | norlutin | norluton | normapause | norpregneninlone | norpregneninolone | norpregneninotone | orlest | ortho 1 35 | ortho 7 7 7 | ortho-novum | ortho-novum 1 35 | ortho-novum 1 50 | ortho-novum 7 7 7 | ovcon | ovysmen | ovysmen 0.5 35 | ovysmen 1 35 | palonyl | perovex | primolut N | primolut-N | proluteasi | synphase | synphasic 28 | tri-norinyl | triella | trinovum | trinovum 21 | utovlan | utovlar
Solid properties (at STP)

density | 1.2 g/cm^3
Units

Chemical identifiers

CAS number | 68-22-4 Beilstein number | 1915671 PubChem CID number | 4536 PubChem SID number | 7847250 SMILES identifier | CC12CCC3C(C1CCC2(C#C)O)CCC4=CC(=O)CCC34 InChI identifier | InChI=1/C20H26O2/c1-3-20(22)11-9-18-17-6-4-13-12-14(21)5-7-15(13)16(17)8-10-19(18, 20)2/h1, 12, 15-18, 22H, 4-11H2, 2H3 InChI key | VIKNJXKGJWUCNN-XGXHKTLJBN EU number | 200-681-6 RTECS number | RC8975000 NSC number | 9564
Toxicity properties

RTECS classes | tumorigen | drug | mutagen | reproductive effector | human data | hormone