Input interpretation
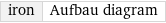
iron | Aufbau diagram
Result
![Build the ground-state orbital diagram for: iron (Fe) Plan: • Look up the abbreviated (condensed) ground electronic configuration. • Use the electronic configuration to identify the outermost electrons. • Determine the energetic ordering of atomic orbital subshells. • Use the following representations: a gray rectangle for an atomic orbital, an up arrow for an alpha (spin-up) spin state and a down arrow for a beta (spin-down) spin state. • Follow the Pauli exclusion principle by representing the first electron in each atomic orbital with an alpha spin state and the second electron with a beta spin state. • Place electrons into orbitals following the Aufbau principle and Hund's rule. The abbreviated ground electronic configuration for iron is: [Ar]4s^23d^6 Electrons in atomic orbitals outside of the argon core, [Ar], are the outermost electrons: 4s^23d^6 Atomic orbital energy usually increases from 1s to 8s in the following order: Follow the Aufbau principle by filling the atomic orbitals with electrons in the following order: 4s < 3d Place two electrons as an alpha-beta pair into the 4s orbital: 4s Place six electrons into the five 3d orbitals with one alpha electron in each of the five orbitals and one beta electron in one of the orbitals: Answer: | | 3d 4s](../image_source/576a64b1a0779df1784f1f51f3216726.png)
Build the ground-state orbital diagram for: iron (Fe) Plan: • Look up the abbreviated (condensed) ground electronic configuration. • Use the electronic configuration to identify the outermost electrons. • Determine the energetic ordering of atomic orbital subshells. • Use the following representations: a gray rectangle for an atomic orbital, an up arrow for an alpha (spin-up) spin state and a down arrow for a beta (spin-down) spin state. • Follow the Pauli exclusion principle by representing the first electron in each atomic orbital with an alpha spin state and the second electron with a beta spin state. • Place electrons into orbitals following the Aufbau principle and Hund's rule. The abbreviated ground electronic configuration for iron is: [Ar]4s^23d^6 Electrons in atomic orbitals outside of the argon core, [Ar], are the outermost electrons: 4s^23d^6 Atomic orbital energy usually increases from 1s to 8s in the following order: Follow the Aufbau principle by filling the atomic orbitals with electrons in the following order: 4s < 3d Place two electrons as an alpha-beta pair into the 4s orbital: 4s Place six electrons into the five 3d orbitals with one alpha electron in each of the five orbitals and one beta electron in one of the orbitals: Answer: | | 3d 4s