Input interpretation

polar solvents | specific heat of vaporization
Summary

median | 0.626 kJ/g highest | 2.23 kJ/g (water) lowest | 0.17 kJ/g (methylene chloride) distribution |
Units

Distribution plots
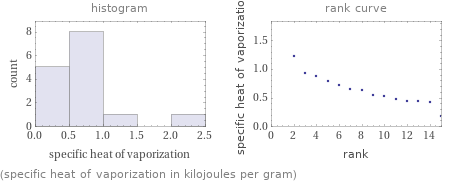
(specific heat of vaporization in kilojoules per gram)
Specific heat of vaporization rankings
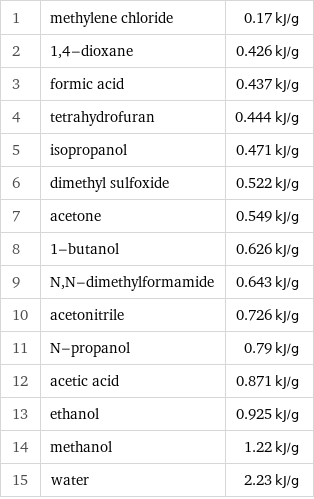
1 | methylene chloride | 0.17 kJ/g 2 | 1, 4-dioxane | 0.426 kJ/g 3 | formic acid | 0.437 kJ/g 4 | tetrahydrofuran | 0.444 kJ/g 5 | isopropanol | 0.471 kJ/g 6 | dimethyl sulfoxide | 0.522 kJ/g 7 | acetone | 0.549 kJ/g 8 | 1-butanol | 0.626 kJ/g 9 | N, N-dimethylformamide | 0.643 kJ/g 10 | acetonitrile | 0.726 kJ/g 11 | N-propanol | 0.79 kJ/g 12 | acetic acid | 0.871 kJ/g 13 | ethanol | 0.925 kJ/g 14 | methanol | 1.22 kJ/g 15 | water | 2.23 kJ/g
Unit conversions for median specific heat of vaporization 0.626 kJ/g

626 J/g (joules per gram)
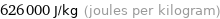
626000 J/kg (joules per kilogram)

150 cal_th/g (thermochemical calories per gram)
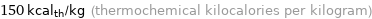
150 kcal_th/kg (thermochemical kilocalories per kilogram)
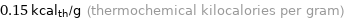
0.15 kcal_th/g (thermochemical kilocalories per gram)

269 BTU_IT/lb (IT British thermal units per pound)