Input interpretation
cosmic ray fission elements
Members
beryllium | boron | lithium
Periodic table location
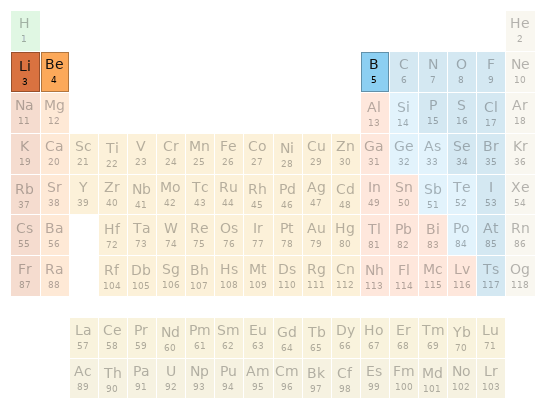
Periodic table location
Images

Images
Basic elemental properties
![| beryllium | boron | lithium atomic symbol | Be | B | Li atomic number | 4 | 5 | 3 short electronic configuration | [He]2s^2 | [He]2s^22p^1 | [He]2s^1 Aufbau diagram | 2s | 2p 2s | 2s block | s | p | s group | 2 | 13 | 1 period | 2 | 2 | 2 atomic mass | 9.0121831 u | 10.81 u | 6.94 u half-life | (stable) | (stable) | (stable)](../image_source/1d455af73bbb16472d7f77deaa13c85e.png)
| beryllium | boron | lithium atomic symbol | Be | B | Li atomic number | 4 | 5 | 3 short electronic configuration | [He]2s^2 | [He]2s^22p^1 | [He]2s^1 Aufbau diagram | 2s | 2p 2s | 2s block | s | p | s group | 2 | 13 | 1 period | 2 | 2 | 2 atomic mass | 9.0121831 u | 10.81 u | 6.94 u half-life | (stable) | (stable) | (stable)
Thermodynamic properties
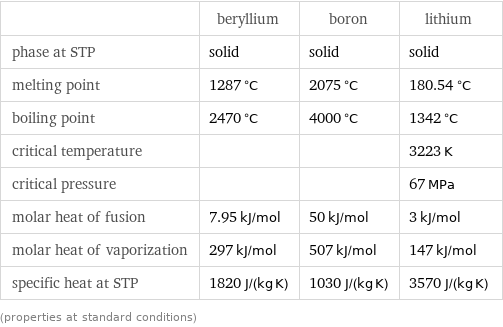
| beryllium | boron | lithium phase at STP | solid | solid | solid melting point | 1287 °C | 2075 °C | 180.54 °C boiling point | 2470 °C | 4000 °C | 1342 °C critical temperature | | | 3223 K critical pressure | | | 67 MPa molar heat of fusion | 7.95 kJ/mol | 50 kJ/mol | 3 kJ/mol molar heat of vaporization | 297 kJ/mol | 507 kJ/mol | 147 kJ/mol specific heat at STP | 1820 J/(kg K) | 1030 J/(kg K) | 3570 J/(kg K) (properties at standard conditions)
Material properties
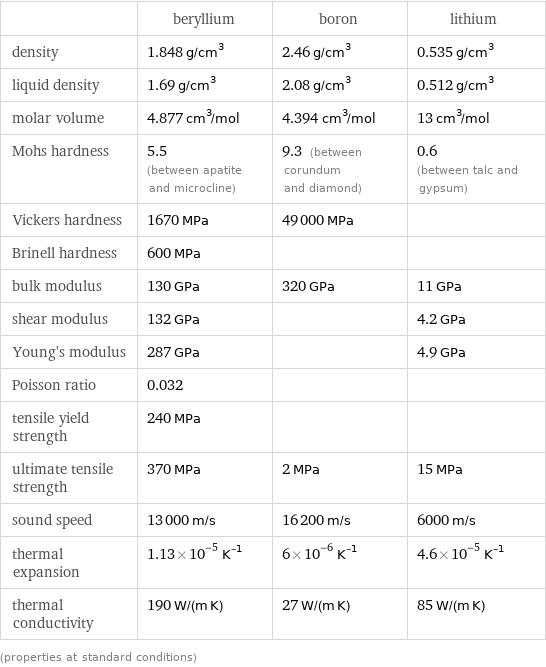
| beryllium | boron | lithium density | 1.848 g/cm^3 | 2.46 g/cm^3 | 0.535 g/cm^3 liquid density | 1.69 g/cm^3 | 2.08 g/cm^3 | 0.512 g/cm^3 molar volume | 4.877 cm^3/mol | 4.394 cm^3/mol | 13 cm^3/mol Mohs hardness | 5.5 (between apatite and microcline) | 9.3 (between corundum and diamond) | 0.6 (between talc and gypsum) Vickers hardness | 1670 MPa | 49000 MPa | Brinell hardness | 600 MPa | | bulk modulus | 130 GPa | 320 GPa | 11 GPa shear modulus | 132 GPa | | 4.2 GPa Young's modulus | 287 GPa | | 4.9 GPa Poisson ratio | 0.032 | | tensile yield strength | 240 MPa | | ultimate tensile strength | 370 MPa | 2 MPa | 15 MPa sound speed | 13000 m/s | 16200 m/s | 6000 m/s thermal expansion | 1.13×10^-5 K^(-1) | 6×10^-6 K^(-1) | 4.6×10^-5 K^(-1) thermal conductivity | 190 W/(m K) | 27 W/(m K) | 85 W/(m K) (properties at standard conditions)
Electromagnetic properties
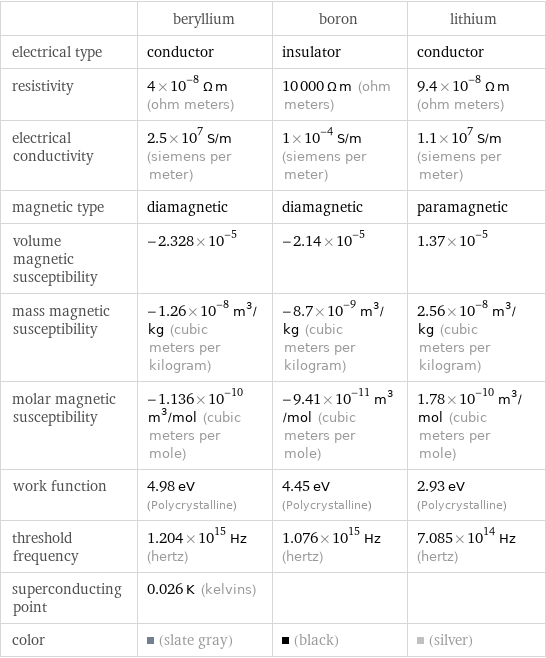
| beryllium | boron | lithium electrical type | conductor | insulator | conductor resistivity | 4×10^-8 Ω m (ohm meters) | 10000 Ω m (ohm meters) | 9.4×10^-8 Ω m (ohm meters) electrical conductivity | 2.5×10^7 S/m (siemens per meter) | 1×10^-4 S/m (siemens per meter) | 1.1×10^7 S/m (siemens per meter) magnetic type | diamagnetic | diamagnetic | paramagnetic volume magnetic susceptibility | -2.328×10^-5 | -2.14×10^-5 | 1.37×10^-5 mass magnetic susceptibility | -1.26×10^-8 m^3/kg (cubic meters per kilogram) | -8.7×10^-9 m^3/kg (cubic meters per kilogram) | 2.56×10^-8 m^3/kg (cubic meters per kilogram) molar magnetic susceptibility | -1.136×10^-10 m^3/mol (cubic meters per mole) | -9.41×10^-11 m^3/mol (cubic meters per mole) | 1.78×10^-10 m^3/mol (cubic meters per mole) work function | 4.98 eV (Polycrystalline) | 4.45 eV (Polycrystalline) | 2.93 eV (Polycrystalline) threshold frequency | 1.204×10^15 Hz (hertz) | 1.076×10^15 Hz (hertz) | 7.085×10^14 Hz (hertz) superconducting point | 0.026 K (kelvins) | | color | (slate gray) | (black) | (silver)
Reactivity
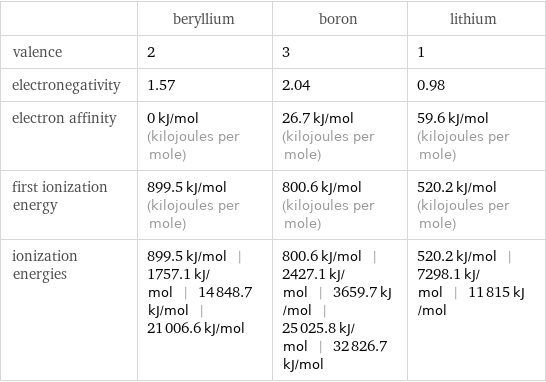
| beryllium | boron | lithium valence | 2 | 3 | 1 electronegativity | 1.57 | 2.04 | 0.98 electron affinity | 0 kJ/mol (kilojoules per mole) | 26.7 kJ/mol (kilojoules per mole) | 59.6 kJ/mol (kilojoules per mole) first ionization energy | 899.5 kJ/mol (kilojoules per mole) | 800.6 kJ/mol (kilojoules per mole) | 520.2 kJ/mol (kilojoules per mole) ionization energies | 899.5 kJ/mol | 1757.1 kJ/mol | 14848.7 kJ/mol | 21006.6 kJ/mol | 800.6 kJ/mol | 2427.1 kJ/mol | 3659.7 kJ/mol | 25025.8 kJ/mol | 32826.7 kJ/mol | 520.2 kJ/mol | 7298.1 kJ/mol | 11815 kJ/mol
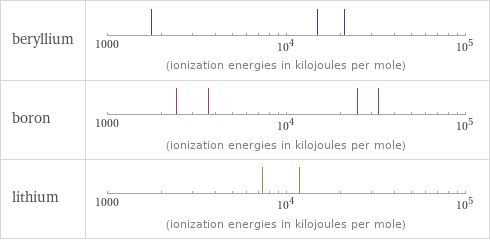
Reactivity
Atomic properties
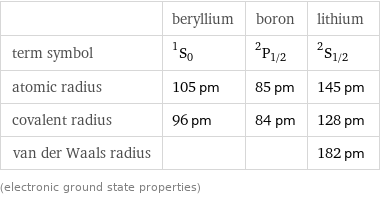
| beryllium | boron | lithium term symbol | ^1S_0 | ^2P_(1/2) | ^2S_(1/2) atomic radius | 105 pm | 85 pm | 145 pm covalent radius | 96 pm | 84 pm | 128 pm van der Waals radius | | | 182 pm (electronic ground state properties)
Abundances
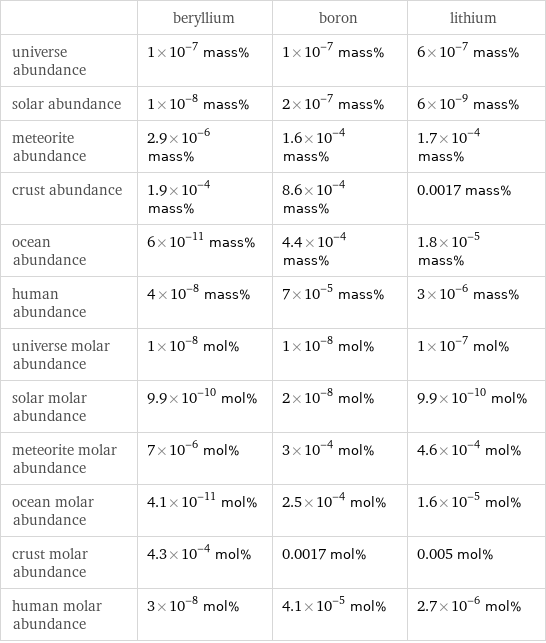
| beryllium | boron | lithium universe abundance | 1×10^-7 mass% | 1×10^-7 mass% | 6×10^-7 mass% solar abundance | 1×10^-8 mass% | 2×10^-7 mass% | 6×10^-9 mass% meteorite abundance | 2.9×10^-6 mass% | 1.6×10^-4 mass% | 1.7×10^-4 mass% crust abundance | 1.9×10^-4 mass% | 8.6×10^-4 mass% | 0.0017 mass% ocean abundance | 6×10^-11 mass% | 4.4×10^-4 mass% | 1.8×10^-5 mass% human abundance | 4×10^-8 mass% | 7×10^-5 mass% | 3×10^-6 mass% universe molar abundance | 1×10^-8 mol% | 1×10^-8 mol% | 1×10^-7 mol% solar molar abundance | 9.9×10^-10 mol% | 2×10^-8 mol% | 9.9×10^-10 mol% meteorite molar abundance | 7×10^-6 mol% | 3×10^-4 mol% | 4.6×10^-4 mol% ocean molar abundance | 4.1×10^-11 mol% | 2.5×10^-4 mol% | 1.6×10^-5 mol% crust molar abundance | 4.3×10^-4 mol% | 0.0017 mol% | 0.005 mol% human molar abundance | 3×10^-8 mol% | 4.1×10^-5 mol% | 2.7×10^-6 mol%
Nuclear properties
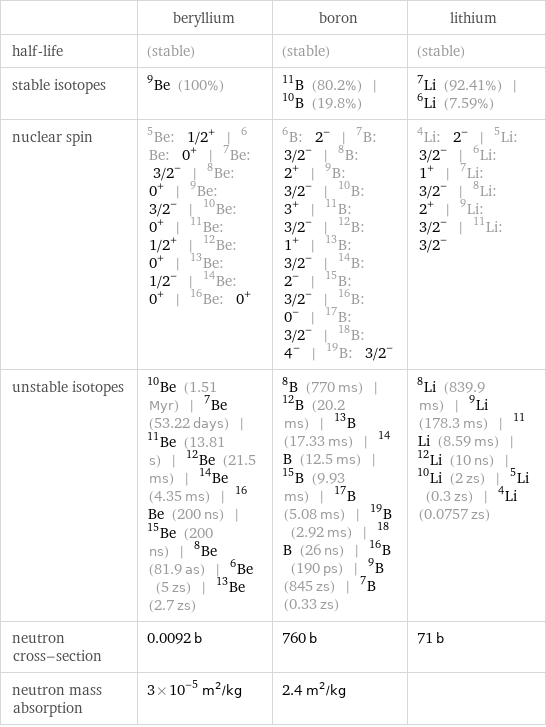
| beryllium | boron | lithium half-life | (stable) | (stable) | (stable) stable isotopes | Be-9 (100%) | B-11 (80.2%) | B-10 (19.8%) | Li-7 (92.41%) | Li-6 (7.59%) nuclear spin | Be-5: 1/2^+ | Be-6: 0^+ | Be-7: 3/2^- | Be-8: 0^+ | Be-9: 3/2^- | Be-10: 0^+ | Be-11: 1/2^+ | Be-12: 0^+ | Be-13: 1/2^- | Be-14: 0^+ | Be-16: 0^+ | B-6: 2^- | B-7: 3/2^- | B-8: 2^+ | B-9: 3/2^- | B-10: 3^+ | B-11: 3/2^- | B-12: 1^+ | B-13: 3/2^- | B-14: 2^- | B-15: 3/2^- | B-16: 0^- | B-17: 3/2^- | B-18: 4^- | B-19: 3/2^- | Li-4: 2^- | Li-5: 3/2^- | Li-6: 1^+ | Li-7: 3/2^- | Li-8: 2^+ | Li-9: 3/2^- | Li-11: 3/2^- unstable isotopes | Be-10 (1.51 Myr) | Be-7 (53.22 days) | Be-11 (13.81 s) | Be-12 (21.5 ms) | Be-14 (4.35 ms) | Be-16 (200 ns) | Be-15 (200 ns) | Be-8 (81.9 as) | Be-6 (5 zs) | Be-13 (2.7 zs) | B-8 (770 ms) | B-12 (20.2 ms) | B-13 (17.33 ms) | B-14 (12.5 ms) | B-15 (9.93 ms) | B-17 (5.08 ms) | B-19 (2.92 ms) | B-18 (26 ns) | B-16 (190 ps) | B-9 (845 zs) | B-7 (0.33 zs) | Li-8 (839.9 ms) | Li-9 (178.3 ms) | Li-11 (8.59 ms) | Li-12 (10 ns) | Li-10 (2 zs) | Li-5 (0.3 zs) | Li-4 (0.0757 zs) neutron cross-section | 0.0092 b | 760 b | 71 b neutron mass absorption | 3×10^-5 m^2/kg | 2.4 m^2/kg |
Identifiers

| beryllium | boron | lithium CAS number | 7440-41-7 | 7440-42-8 | 7439-93-2 PubChem CID number | 5460467 | 5462311 | 3028194