Input interpretation
osmium | common compound names
Result

osmium(III) chloride hydrate | ammonium hexachloroosmate(IV) | potassium hexachloroosmate(IV) | potassium osmate(VI) dihydrate | triosmium dodecacarbonyl | sodium hexachloroosmate(IV) hydrate | osmium(III) chloride | pentaammine(dinitrogen)osmium(II) chloride | pentaammine(trifluoromethanesulfonato)osmium(III) triflate | bis(pentamethylcyclopentadienyl)osmium(II) | potassium nitridotrioxoosmate(VIII) | dodecacarbonyltetra-μ-hydridotetraosmium | osmium tetroxide (total: 13)
Basic properties
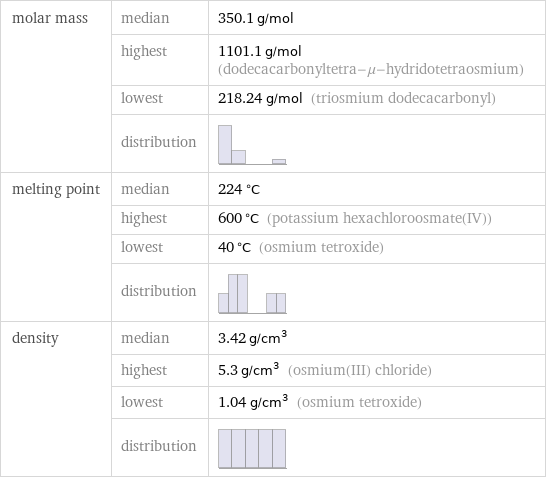
molar mass | median | 350.1 g/mol | highest | 1101.1 g/mol (dodecacarbonyltetra-μ-hydridotetraosmium) | lowest | 218.24 g/mol (triosmium dodecacarbonyl) | distribution | melting point | median | 224 °C | highest | 600 °C (potassium hexachloroosmate(IV)) | lowest | 40 °C (osmium tetroxide) | distribution | density | median | 3.42 g/cm^3 | highest | 5.3 g/cm^3 (osmium(III) chloride) | lowest | 1.04 g/cm^3 (osmium tetroxide) | distribution |
Units
