Input interpretation

actinides | specific heat at STP
Summary

median | 117 J/(kg K) highest | 120 J/(kg K) (actinium) lowest | 99.1 J/(kg K) (protactinium) distribution | | (based on 4 values; 11 unavailable)
Entities with missing values

neptunium | plutonium | americium | curium | berkelium | californium | einsteinium | fermium | mendelevium | nobelium | lawrencium (total: 11)
Specific heat at STP rankings

1 | protactinium | 99.1 J/(kg K) 2 | uranium | 116 J/(kg K) 3 | thorium | 118 J/(kg K) 4 | actinium | 120 J/(kg K) (based on 4 values; 11 unavailable)
Unit conversions for median specific heat at STP 117 J/(kg K)
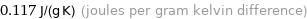
0.117 J/(g K) (joules per gram kelvin difference)
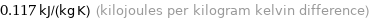
0.117 kJ/(kg K) (kilojoules per kilogram kelvin difference)
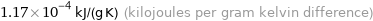
1.17×10^-4 kJ/(g K) (kilojoules per gram kelvin difference)

117 J/(kg °C) (joules per kilogram degree Celsius difference)
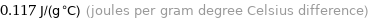
0.117 J/(g °C) (joules per gram degree Celsius difference)

0.0279 BTU_IT/(lb °F) (IT British thermal units per pound degree Fahrenheit difference)
Comparisons for median specific heat at STP 117 J/(kg K)
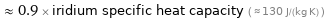
≈ 0.9 × iridium specific heat capacity ( ≈ 130 J/(kg K) )
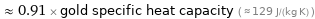
≈ 0.91 × gold specific heat capacity ( ≈ 129 J/(kg K) )
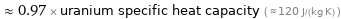
≈ 0.97 × uranium specific heat capacity ( ≈ 120 J/(kg K) )
Thermodynamic properties
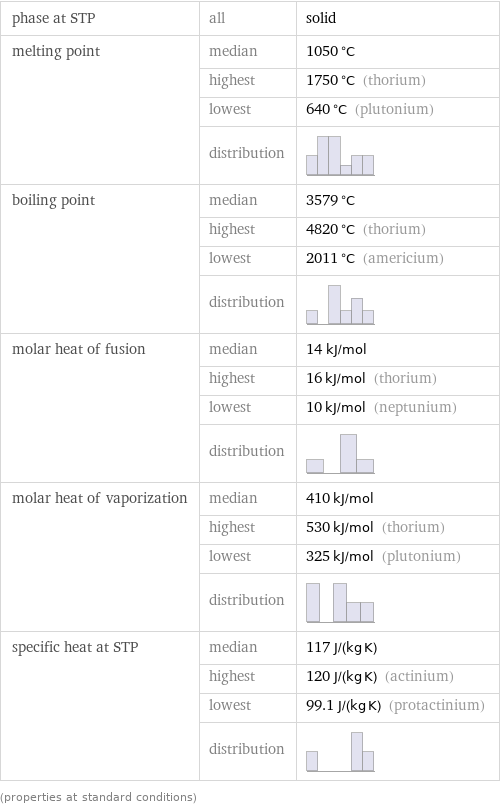
phase at STP | all | solid melting point | median | 1050 °C | highest | 1750 °C (thorium) | lowest | 640 °C (plutonium) | distribution | boiling point | median | 3579 °C | highest | 4820 °C (thorium) | lowest | 2011 °C (americium) | distribution | molar heat of fusion | median | 14 kJ/mol | highest | 16 kJ/mol (thorium) | lowest | 10 kJ/mol (neptunium) | distribution | molar heat of vaporization | median | 410 kJ/mol | highest | 530 kJ/mol (thorium) | lowest | 325 kJ/mol (plutonium) | distribution | specific heat at STP | median | 117 J/(kg K) | highest | 120 J/(kg K) (actinium) | lowest | 99.1 J/(kg K) (protactinium) | distribution | (properties at standard conditions)