Input interpretation
calcium fluorophosphate | magnesium phosphate hydrate
Chemical names and formulas
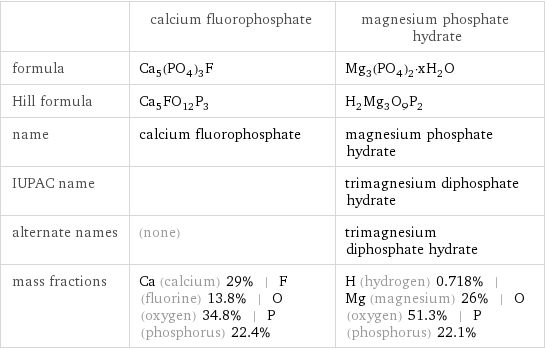
| calcium fluorophosphate | magnesium phosphate hydrate formula | Ca_5(PO_4)_3F | Mg_3(PO_4)_2·xH_2O Hill formula | Ca_5FO_12P_3 | H_2Mg_3O_9P_2 name | calcium fluorophosphate | magnesium phosphate hydrate IUPAC name | | trimagnesium diphosphate hydrate alternate names | (none) | trimagnesium diphosphate hydrate mass fractions | Ca (calcium) 29% | F (fluorine) 13.8% | O (oxygen) 34.8% | P (phosphorus) 22.4% | H (hydrogen) 0.718% | Mg (magnesium) 26% | O (oxygen) 51.3% | P (phosphorus) 22.1%
Structure diagrams
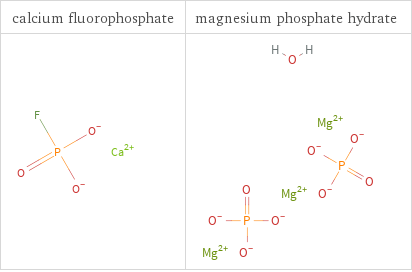
Structure diagrams
Basic properties
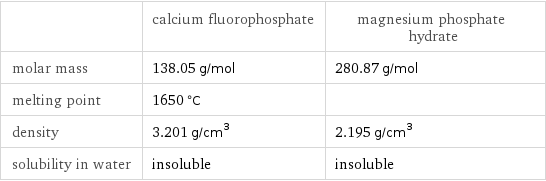
| calcium fluorophosphate | magnesium phosphate hydrate molar mass | 138.05 g/mol | 280.87 g/mol melting point | 1650 °C | density | 3.201 g/cm^3 | 2.195 g/cm^3 solubility in water | insoluble | insoluble
Units
