Input interpretation
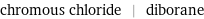
chromous chloride | diborane
Chemical names and formulas
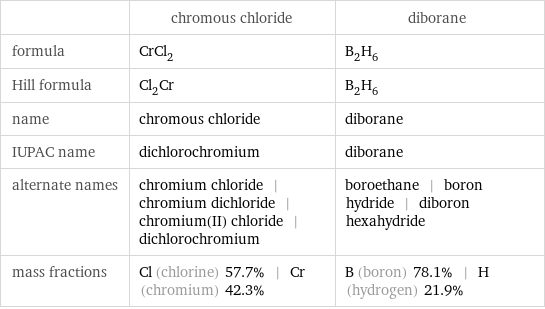
| chromous chloride | diborane formula | CrCl_2 | B_2H_6 Hill formula | Cl_2Cr | B_2H_6 name | chromous chloride | diborane IUPAC name | dichlorochromium | diborane alternate names | chromium chloride | chromium dichloride | chromium(II) chloride | dichlorochromium | boroethane | boron hydride | diboron hexahydride mass fractions | Cl (chlorine) 57.7% | Cr (chromium) 42.3% | B (boron) 78.1% | H (hydrogen) 21.9%
Structure diagrams
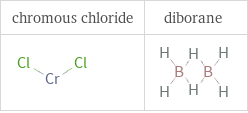
Structure diagrams
3D structure
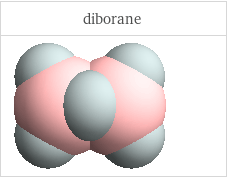
3D structure
Basic properties
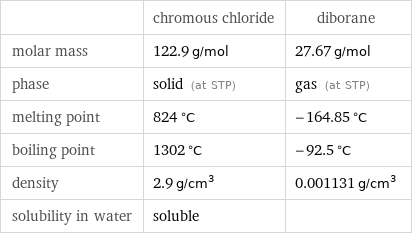
| chromous chloride | diborane molar mass | 122.9 g/mol | 27.67 g/mol phase | solid (at STP) | gas (at STP) melting point | 824 °C | -164.85 °C boiling point | 1302 °C | -92.5 °C density | 2.9 g/cm^3 | 0.001131 g/cm^3 solubility in water | soluble |
Units

Gas properties
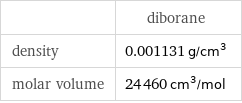
| diborane density | 0.001131 g/cm^3 molar volume | 24460 cm^3/mol
Units

Thermodynamic properties
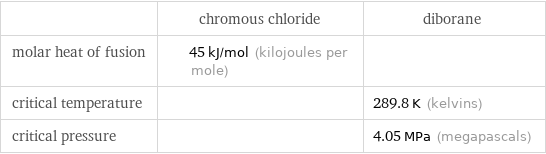
| chromous chloride | diborane molar heat of fusion | 45 kJ/mol (kilojoules per mole) | critical temperature | | 289.8 K (kelvins) critical pressure | | 4.05 MPa (megapascals)